
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11, Problem 43P
A synthesis of the
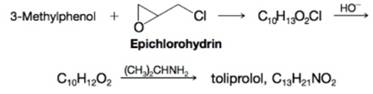
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In the mid-1930s a substance was isolated from a fungus that is a parasite of ryes and other grasses. This alkaloid, lysergic acid, has
been of great interest to chemists because of its strange, dramatic action on the human mind. Many derivatives of lysergic acid are
known, some with medicinal applications. Perhaps the best known derivative of lysergic acid is the potent hallucinogen lysergic acid
diethylamide (LSD):
మగవా జి
N-H
LSD
(CH25N;O)
Like other alkaloids, LSD is a weak base, with Kp = 7.6 × 107. What is the pH of a 0.94 M solution of LSD?
pH =
Predict the products obtained from the reaction of triolein with the following reagents.(a) NaOH in water
Explain the following statements. You must use chemical equations to justify your
explanation.
(ii)
(I)
Phenol is more acidic than cyclohexanol.
Chapter 11 Solutions
Organic Chemistry
Ch. 11 - Prob. 1PPCh. 11 - Practice Problem 11.2 Give bond-line formulas and...Ch. 11 - Prob. 3PPCh. 11 - Prob. 4PPCh. 11 - Prob. 5PPCh. 11 - Prob. 6PPCh. 11 - Prob. 7PPCh. 11 - Practice Problem 11.8 Show how you would prepare...Ch. 11 - Prob. 9PPCh. 11 - Prob. 10PP
Ch. 11 - Practice Problem 11.11
An exception to what is...Ch. 11 - Prob. 12PPCh. 11 - Prob. 13PPCh. 11 - Prob. 14PPCh. 11 - Prob. 15PPCh. 11 - Prob. 16PPCh. 11 - Prob. 17PPCh. 11 - Prob. 18PPCh. 11 - Practice Problem 11.19
Propose structures for each...Ch. 11 - Prob. 20PPCh. 11 - Prob. 21PPCh. 11 - Prob. 22PPCh. 11 - Prob. 23PPCh. 11 - Prob. 24PPCh. 11 - Give an IUPAC substitutive name for each of the...Ch. 11 - Prob. 26PCh. 11 - Prob. 27PCh. 11 - Prob. 28PCh. 11 - Prob. 29PCh. 11 - 11.30. Show how you might prepare 2-bromobutane...Ch. 11 - Prob. 31PCh. 11 - Prob. 32PCh. 11 - Prob. 33PCh. 11 - Considering A-L to represent the major products...Ch. 11 - Write structures for the products that would be...Ch. 11 - Prob. 36PCh. 11 - Provide the reagents necessary for the following...Ch. 11 - 11.38. Predict the major product from each of the...Ch. 11 - Predict the products from each of the following...Ch. 11 - Provide the reagents necessary to accomplish the...Ch. 11 - 11.41. Provide reagents that would accomplish the...Ch. 11 - Prob. 42PCh. 11 - 11.43. A synthesis of the -receptor blocker called...Ch. 11 - Prob. 44PCh. 11 - Prob. 45PCh. 11 - 11.46. For each of the following, write a...Ch. 11 - 11.47. Vicinal halo alcohols (halohydrins) can be...Ch. 11 - Prob. 48PCh. 11 - Prob. 49PCh. 11 - Prob. 50PCh. 11 - Prob. 51PCh. 11 - Prob. 52PCh. 11 - Outlined below is a synthesis of the gypsy moth...Ch. 11 - Prob. 54PCh. 11 - Prob. 55PCh. 11 - Prob. 56PCh. 11 - 11.57. When the 3-bromo-2-butanol with the...Ch. 11 - 11.58. Reaction of an alcohol with thionyl...Ch. 11 - Prob. 59PCh. 11 - Prob. 60PCh. 11 - Prob. 1LGPCh. 11 - Prob. 2LGPCh. 11 - Synthesize the compound shown below from...
Additional Science Textbook Solutions
Find more solutions based on key concepts
a. Prepare a molecular orbital energy-level diagram for NO showing clearly how the atomic orbitals interact to ...
Inorganic Chemistry
12.1 Give the IUPAC name for each of the following:
a. CH3-CH2-OH
b.
c.
d.
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
The evidence that caused Rutherford to change Thomson’s plum pudding model to nuclear model must be explained. ...
Living By Chemistry: First Edition Textbook
Practice Problem 13.16
Diels–Alder reactions also take place with triple-bonded (acetylenic) dienophiles. Whic...
Organic Chemistry
12.1 Give the IUPAC name for each of the following:
a. CH3-CH2-OH
b.
c.
d.
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Phenylacetone can form two different enols.(a) Show the structures of these enols.(b) Predict which enol will be present in the larger concentration at equilibrium.(c) Propose mechanisms for the formation of the two enols in acid and in basearrow_forward(b) Suggest a reasonable biosynthesis for the naturally occurring alkaloid coniine (isolated from hemlock), starting from octanoic acid. Coniinearrow_forwardBorazole is an unusually stable cyclic compound. Propose a structure forborazole, and explain why it is aromatic.arrow_forward
- Primary amines can also be prepared by the reaction of an alkyl halide with azide ion, followed by catalytic hydrogenation. What advantage do this method and the Gabriel synthesis have over the synthesis of a primaryamine using an alkyl halide and ammonia?arrow_forwardWrite a structural formula for each of the following compounds: (a) m-Chlorobenzoyl chloride (b) Trifluoroacetic anhydride (c) cis-1,2-Cyclopropanedicarboxylic anhydride (d) Ethyl cycloheptanecarboxylate (e) 1-Phenylethyl acetate (f) 2-Phenylethyl acetate (g) p-Ethylbenzamide (h) N-Ethylbenzamide (i) 2-Methylhexanenitrilearrow_forwardWhich is the stronger acid in each of the following pairs? Explain your reasoning. (a) Phenol or p-hydroxybenzaldehyde (b) m-Cyanophenol or p-cyanophenol (c) o-Fluorophenol or p-fluorophenolarrow_forward
- Discovery of the antibiotic sulphanilamide led to rapid development of a large number of structurally related sulphonamides. Some of these were useful leads to compounds with other medicinal properties. Amongst these, sulfasalazine was active in the treatment of ulcerative colitis, a potentially fatal disease of the colon. As a medicinal chemist, you are about to carry out the synthesis of sulfasalazine, starting from aniline. Give the chemical names and draw the chemical structures of the reagents you will need to use for the all the steps marked (a)-(d). Aniline HO₂C HO (a) N=N- ACHN Ac represents CH3CO Sulfasalazine SO,NH S0,C1 N (b) (d) H₂N- SO,NH (c) N [Oro] SO,NH N Step (a) in question 5 above results in the para product only. Explain why this occurs.arrow_forwardQuinapril (trade name Accupril) is used to treat high blood pressure andcongestive heart failure. One step in the synthesis of quinapril involvesreaction of the racemic alkyl bromide A with a single enantiomer of theamino ester B. Given the structure of quinapril, which one of these two products isneeded to synthesize the drug?arrow_forwardFollowing is an outline of a synthesis of the bronchodilator carbuterol, a beta-2 adrenergic blocker with high selectivity for airway smooth muscle receptors. Q.Suggest a structural relationship between carbuterol and ephedrinearrow_forward
- Give the products expected when the following tertiary amines are treated with a peroxyacid and heated.(a) N,N-dimethylhexan-2-aminearrow_forwardFollowing is a synthesis for toremifene, a nonsteroidal estrogen antagonist whose structure is closely related to that of tamoxifen. (a) This synthesis makes use of two blocking groups, the benzyl (Bn) group and the tetrahydropyranyl (THP) group. Draw a structural formula of each group and describe the experimental conditions under which it is attached and removed. (b) Discuss the chemical logic behind the use of each blocking group in this synthesis. (c) Propose a mechanism for the conversion of D to E. (d) Propose a mechanism for the conversion of F to toremifene. (e) Is toremifene chiral? If so, which of the possible stereoisomers are formed in this synthesis?arrow_forwardFollowing are the steps in the industrial synthesis of glycerin. Provide structures for all intermediate compounds (AD) and describe the type of mechanism by which each is formed.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY