
Concept explainers
a)
Interpretation:
A reaction that leads to the formation of triglyceride, starting with glycerol and carboxylic acids has to be suggested.
Concept introduction:
Ester formation reaction: Reaction of alcohol and carboxylic acid using acid catalyst results the ester formation with the elimination of water molecule.

a)
Explanation of Solution
The hydroxyl group act as nucleophile and the carboxylic group act as electrophile in presence of acid catalyst; the nucleophile attack at electrophilic carbon of carboxylic acid leads to the formation of ester with the elimination of water molecule.
Mechanism of condensation reaction:
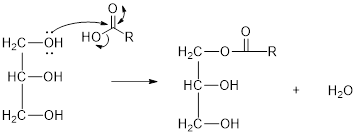
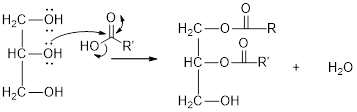
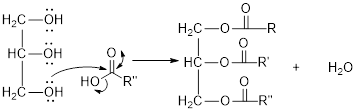
As shown above, the successive steps lead to the formation of triglycerides containing three ester group with the elimination of three water molecules.
b)
Interpretation:
An equation for the base hydrolysis of ester has to be written.
Concept introduction:
Ester formation reaction: Reaction of alcohol and carboxylic acid using acid catalyst results the ester formation with the elimination of water molecule.

b)
Explanation of Solution
The hydroxyl group acts as nucleophile and the carbonyl carbon act as electrophile; the nucleophile attack at electrophilic carbon of ester leads to the formation of alcohol with the elimination of fatty acid salts (soap).
Base hydrolysis of Esters:
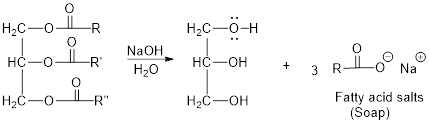
c)
Interpretation:
Difference between fats and oils has to be explained.
Concept introduction:
Melting point: At temperature begins the solid to melt.
Unsaturation bonds: The presence of double or triple bonds in the molecules.
c)
Explanation of Solution
The presence of unsaturated bonds in the molecules tight close packing will be less due to bend of double bonds and the intermolecular attraction between them is less and less energy is required to overcome the interaction. More the double bonds lower the intermolecular interaction. Hence, the melting point decreases.
d)
Interpretation:
Reagent and catalyst used in hydrogenation process has to be identified.
Concept introduction:
Hydrogenation of
Homogeneous catalyst: Catalyst used is in same phase as the reactants.
Heterogeneous catalyst: Catalyst used is in different phase as the reactants.
d)
Explanation of Solution
Liquid oil is obtained from plants, having double bonds the presence of reactive double bond is converted into single bonds in order to solidify. Hydrogenation of double bonds is the process in which hydrogen molecule is added across the double bond forming alkane product. The alkane is highly facilitated for close packing and solidifies the oil.
Reaction carried out is hydrogenation reaction; hydrogen molecule is the reagent used in presence of either heterogeneous or homogeneous catalyst.
e)
Interpretation:
Iodine number has to be calculated.
Concept introduction:
Iodine number: number of grams of Iodine that react with given quantity of oil is called Iodine number.
Number of moles = Molarity
e)
Explanation of Solution
Given: molarity of
Number of moles of
The mol ratio between
Number of grams of
The iodine number is the number of grams of iodine that reacts with 100 g of corn oil.
Hence, Iodine number calculated is 123
Want to see more full solutions like this?
Chapter 11 Solutions
General Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





