
Interpretation:
The percentage of a and β phase in the phase diagram needs to be determined.
Concept introduction:
Alloy is a combination of two or more metals with another element or metal to form a new metal. The formed metal by combining two metals has different properties from pure metal like increased hardness, toughness, surface roughness;etc.an alloy may be a solid solution or combination of metallic phase. Alloys are having different metallic bonding character.
Answer to Problem 11.27P
At,
At,
At,
At,
At,
Explanation of Solution
Given:
5 percent weight of Si alloy at temperature
Calculation:
The given phase diagram is as follows:
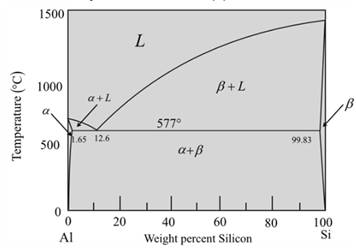
The given diagram is a graph of plotted against temperature and weight percent of silicon.
So, to calculate percent of a-phase and liquidphase first,
Draw a vertical linefrom alloy composition Al-5wt%Si
At temperature
Now for liquid phase,
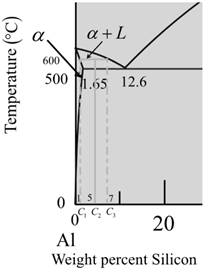
From the given diagram is a graph plotted verses temperature weight percentage of silicon.
For temperature
The percentage of liquid phase is calculated as,
Where,
Put,
Now for a-phase,
So, percentage of phase at temperature
Percent of phase at calculate percent of as,
Where,
Put,
Therefore, weight percentage is,
So, the percentage of phase at
Now for liquid phase,
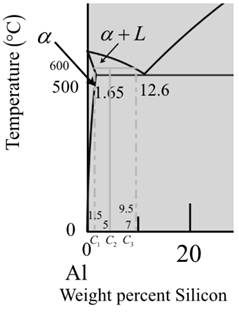
The diagram is a graph plotted against temperature and weight percent of silicon.
Calculate the percent of liquid phase by the formula,
Where,
Put,
Therefore, weight percentage is,
Now, calculate the percent for a-phase,
Where,
Put,
Therefore, at
Now for temperature at
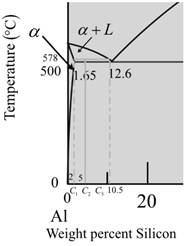
The given diagram is a graph plotted against temperature and weight percent of silicon.
Calculate the percentage of liquid phase as,
Where,
Put,
Now, calculate the percentage of a-phase.
Where,
Put,
Therefore, at
Now for temperature
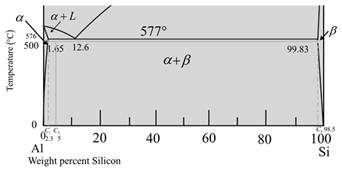
The given diagram is a graph plotted against temperature and weight percent of silicon.
Now calculate the percentage of phase
Therefore, the percentage of β-phase,
Where,
Put,
Calculate the percentage of a- phase.
Where,
Put,
Therefore, at
Now for temperature
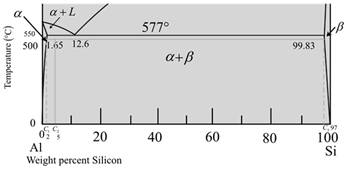
The given diagram is a graph plotted against temperature and weight percent of silicon.
Now calculate the percentage of phase
Therefore, the percentage of β-phase,
Where,
Put,
Calculate the percentage of a- phase.
Where,
Put,
Therefore, at
At,
At,
At,
At,
At,
Want to see more full solutions like this?
Chapter 11 Solutions
Essentials Of Materials Science And Engineering
 MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc
MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage,
Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage, Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning
Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION
Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON
Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY
Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY





