
Chemistry In Context
9th Edition
ISBN: 9781259638145
Author: Fahlman, Bradley D., Purvis-roberts, Kathleen, Kirk, John S., Bentley, Anne K., Daubenmire, Patrick L., ELLIS, Jamie P., Mury, Michael T., American Chemical Society
Publisher: Mcgraw-hill Education,
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 10, Problem 10.1YT
Interpretation Introduction
Interpretation:
To each food taste is responds to which part of your mouth has to be identified.
Expert Solution & Answer
Explanation of Solution
Respond with the traditional diagram with sour on the sides of the tongue, bitter on the back, and salty/sweet on the front. Some students may have very sensitive palettes and some may have a very dulled sense of taste.
The traditional diagram:
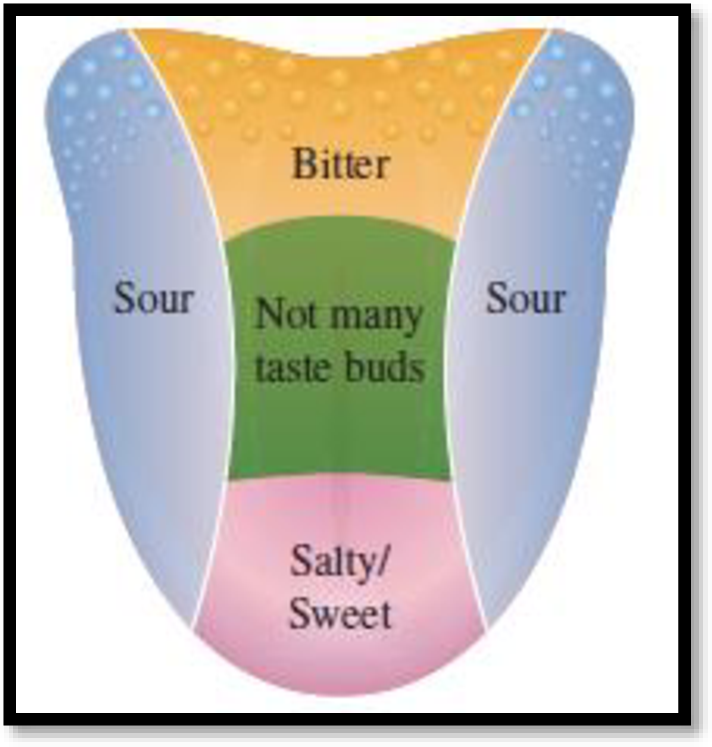
Figure 1
Want to see more full solutions like this?
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts!
Students have asked these similar questions
When you are calculating water hardness, all the compounds in the water are considered to be -------- ?
Select one:
Limestone
CaCO3
Hydrates
MgCO3
The content of ethanol or alcohol for alcoholic beverages is measured with alcohol proof. A 750 mL bottle of vodka is labeled as 60 proof. What is its alcohol content?
Let the ED50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz.
If the ED50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz?
235 mg
470 mg
32,900 mg
35,000,000 mg
Chapter 10 Solutions
Chemistry In Context
Ch. 10.2 - Prob. 10.4YTCh. 10.3 - Prob. 10.5YTCh. 10.4 - You need to make 100 cookies for your school bake...Ch. 10.4 - Prob. 10.7YTCh. 10.4 - Skill Building Apple Pie Creation from Metric...Ch. 10.4 - Prob. 10.9YTCh. 10.5 - Prob. 10.10YTCh. 10.5 - Prob. 10.11YTCh. 10.6 - Scientific Principles The Sustainability of...Ch. 10.6 - Prob. 10.13YT
Ch. 10.7 - Prob. 10.14YTCh. 10.7 - Prob. 10.15YTCh. 10.9 - Prob. 10.18YTCh. 10.9 - Prob. 10.19YTCh. 10.9 - Maybe you have been to a party or outdoor barbeque...Ch. 10.10 - Using Henrys Law, compare the concentration of...Ch. 10 - Prob. 10.1YTCh. 10 - Prob. 10.2YTCh. 10 - Prob. 1QCh. 10 - Prob. 3QCh. 10 - Prob. 5QCh. 10 - Prob. 6QCh. 10 - Prob. 7QCh. 10 - Prob. 8QCh. 10 - Prob. 9QCh. 10 - Prob. 10QCh. 10 - Prob. 12QCh. 10 - Hikers often complain that it is difficult to make...Ch. 10 - Prob. 15QCh. 10 - Prob. 16QCh. 10 - Prob. 18QCh. 10 - Suggest which of the following molecules will...Ch. 10 - What is the name of the substance responsible for...Ch. 10 - Describe why silver cookware tarnishes, especially...Ch. 10 - Prob. 22QCh. 10 - Prob. 23QCh. 10 - Prob. 24QCh. 10 - Prob. 25QCh. 10 - Prob. 26QCh. 10 - One section in this chapter described the Maillard...Ch. 10 - Prob. 28QCh. 10 - In the coffee industry, equipment ranging from...Ch. 10 - Prob. 30QCh. 10 - Prob. 31Q
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Apple cinnamon Glade air freshener has two fragrance molecules in it. The 'apple' molecule has a molecular weight of 400 grams per mole, while the 'cinnamon' molecule has a molecular weight of 170 grams per mole. You're sitting at the back of the room and have an extremely sensitive nose. If I sprayed apple cinnamon Glade air freshener at the opposite side of the room, which scent would you smell first? Explain your answer.arrow_forwardi could use some help with this question?arrow_forwardCompounds used in pharmacology experiments are often initially made as stock solutions. Stock solutions can then be diluted to the desired final concentration. For instance, take Serotonin (5-HT) which was initially made at a 15 mM stock concentration. If an experiment requires 85 mL of Serotonin at a concentration of 5 μM. What volume of stock solution needs to be diluted? Show your workings.What micropipette would you use to measure this volume?arrow_forward
- What is the concentration of the AR stock solution in ppm? Using the molar mass of AR (496.42 g/mol), and that 1 ppm = 1 mg/L, convert this ppm concentration to μM. Assume the density of the solution is 1.000 g/mL. Concentration of AR Stock Solution (ppm) Concentration of AR Stock Solution (μM) 21.22 Unrounded 3 Rounded 3arrow_forwardCalculations are based off of my data table. I'm not sure how to answer the questions. 1.Based on the results of your experiment, do you have hard or soft water? Does this match the data for your area? Briefly explain your answer. 2.How much calcium would you ingest by drinking one 8 oz glass of your tap water? Show all calculations. 3.What percentage of the recommended daily dose of calcium (1,150 mg/day) does 1.0 L of your water provide? Show all calculations. 4. A complexometric titration can also be used to determine the amount of calcium in milk. The calcium concentration in milk is typically 1,200 mg/L. How would you alter the procedure used in this experiment to determine milk’s calcium content?arrow_forwardI need to make a 500ml solution with 7.49 ug/ml linoleic acid and I have 1.07 g/ml linoleic acid to use. How much linoleic acid do I need to make my 500ml solution at said concentration?arrow_forward
- Direction: Below is a sample dosing table, similar to one you would find on a Drug Facts label. Use the table as well as your knowledge about medicine safety to answer the questions below. Children under 6 years of age ¦ Ask a doctor Children 6 to under 12 years of age 2.5 mL (½ teaspoonful) two times per day; do not give more than 5 mL (1 teaspoonful) in 24 hours Adults and children 12 years of age and over 5 mL (1 teaspoonful) two times per day; do not take more than 10 mL (2 teaspoonful) in 24 hours Adults 65 years of age and 5 ml (1 teaspoonful) two times per day over Guide Questions 1. Olivia is 12 years old, and her parents gave her a first dose of this medicine at 8 a.m. They gave her a second dose at 3 p.m. the same day. By the evening, she is still not feeling better. Based on the table above, when can Olivia's parents give her another dose of this medicine? 2. What might happen if someone used a kitchen spoon to measure out a dose of this medicine? 3. Why do you think doses…arrow_forwardIf a total of 125 mL of a 10% solution is diluted to 500 mL in a 500-mL volumetric flask, what is the concentration of the resulting new solution? You are asked to make a 1/2 dilution using 1.5 mL of serum. How much diluent do you need to use? If you make a five-tube twofold dilution using 2 mL of serum, what is the concentration of serum in tube #4?arrow_forwardJust answer the 4th item. The substances are acetone and alcohol.arrow_forward
- A person’s hemoglobin concentration measures 13 grams per dL of blood. Convert this concentration to g/L of blood. Now express this patient’s hemoglobin concentration in mM.arrow_forwardSolution 1 is diluted by adding 519 microliters of Solution 1 to 5 mL of Solution 2. What is the dilution of Solution 1? Report your answer in standard decimal notation rounded to 2 decimal places. Include trailing zeros so that two decimal points are reported. For example, 500 μL added to 5 mL would be a 0.09 dilution or 50 μL added to 4.5 mL would be a 0.10 dilution.arrow_forwardnormal range of serum glucose is 90-110 mg/dl, express this in mmol/Larrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
