
HEAT+MASS TRANSFER:FUND.+APPL.
6th Edition
ISBN: 9780073398198
Author: CENGEL
Publisher: RENT MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 16P
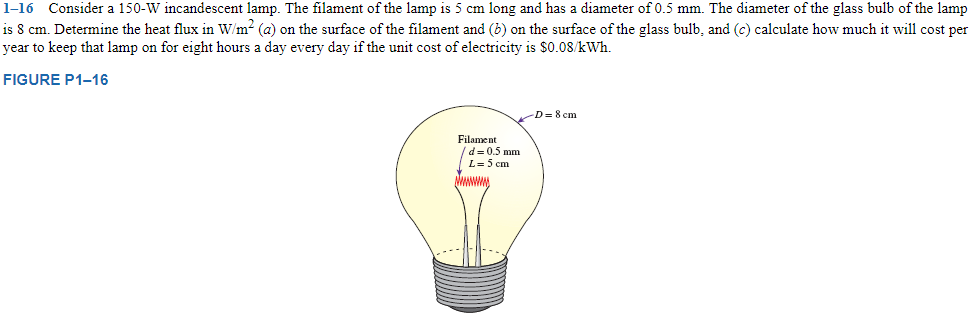
Consider a 150-W incandescent lamp. The filament of the lamp is 5 cm long and has a diameter of 0.5 mm. The diameter of the glass bulb of the lamp is 8 cm. Determine the heat flux in W/m2 (a) on the surface of the filament and (b) on the surface of the glass bulb, and (c) calculate how much it will cost per year to keep that lamp on for eight hours a day every day if the unit cost of electricity if $0.08/kWh.
FIGURE P1-16
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
H.W6
Determine the largest weight
W that can be supported by
two wires shown in Fig. P109.
The stress in either wire is not
to exceed 30 ksi. The cross-
sectional areas of wires AB
and AC are 0.4 in2 and 0.5
in2, respectively.
50°
30°
W
Find equation of motion and natural frequency for the system shown in fig. by energy
method.
H.W2// For the system Fig below find
1-F.B.D
2-Eq.of motion
8wn
4-0 (5)
m. Jo
m
2. Read the following Vernier caliper measurements. (The scales have been
enlarged for easier reading.) The Vernier caliper is calibrated in metric units.
(a)
0 1
2
3
4 5
سلسلسله
(b)
1
2
3
4 5 6
سلسل
(c)
1 23456
(d)
1 2 3
4 5 6
سلسلس
Chapter 1 Solutions
HEAT+MASS TRANSFER:FUND.+APPL.
Ch. 1 - How does the science of heat transfer differ from...Ch. 1 - What is the driving force for (a) heat transfer,...Ch. 1 - How do rating problems in heat transfer differ...Ch. 1 - What is the difference between the analytical and...Ch. 1 - What is the importance of modeling in engineering?...Ch. 1 - When modeling an engineering process, how is the...Ch. 1 - On a hot summer day, a student turns his fan on...Ch. 1 - Consider two identical rooms, one with a...Ch. 1 - Prob. 9CPCh. 1 - Prob. 10CP
Ch. 1 - Prob. 11CPCh. 1 - An ideal gas is heated from 50C to 80C (a) at...Ch. 1 - What is heat flux? How is it related to the heat...Ch. 1 - What are the mechanisms of energy transfer to a...Ch. 1 - A logic chip used in a computer dissipates 3 W of...Ch. 1 - Consider a 150-W incandescent lamp. The filament...Ch. 1 - A 15-cm-diameter aluminum ball is to be heated...Ch. 1 - A 60-gallon water heated is initially filled with...Ch. 1 - Prob. 19PCh. 1 - Prob. 20PCh. 1 - Prob. 21PCh. 1 - Prob. 22PCh. 1 - Prob. 23PCh. 1 - Prob. 24PCh. 1 - Prob. 25PCh. 1 - Prob. 26PCh. 1 - A 5-m6-m8-m room is to be heated by an electrical...Ch. 1 - Prob. 28PCh. 1 - Air enters the duct of an air-conditioning system...Ch. 1 - Prob. 30PCh. 1 - Define thermal conductivity, and explain its...Ch. 1 - Which is a better heat conductor, diamond or...Ch. 1 - How do the thermal conductivity of gases and...Ch. 1 - Why is the thermal conductivity of superinsulation...Ch. 1 - Why do we characterize the heat conduction ability...Ch. 1 - What are the mechanisms of heat transfer? How are...Ch. 1 - Write down the expression for the physical laws...Ch. 1 - How does heat conduction differ from convection?Ch. 1 - Does any of the energy of the sun reach the earth...Ch. 1 - How does forced convection differ from natural...Ch. 1 - What is the physical mechanism of heat conduction...Ch. 1 - Consider heat transfer a windowless wall of house...Ch. 1 - Consider heat loss through two walls of house on a...Ch. 1 - Consider two houses that are identical except that...Ch. 1 - Consider two walls of a house that are identical...Ch. 1 - Define emissivity and absorptivity. What is...Ch. 1 - What is a blackbody? How do real bodies differ...Ch. 1 - A wood slab with a thickness 0.05 m is subjected...Ch. 1 - Prob. 49PCh. 1 - Prob. 50EPCh. 1 - The inner and outer surfaces of a 0.5-cm thick...Ch. 1 - Prob. 52PCh. 1 - Prob. 53PCh. 1 - The north wall of an electrically heated home is...Ch. 1 - Prob. 55PCh. 1 - Prob. 56PCh. 1 - Prob. 57PCh. 1 - A concreate wall a surface area of 20 m2 and a...Ch. 1 - Prob. 59PCh. 1 - Prob. 60PCh. 1 - Prob. 61PCh. 1 - Prob. 62EPCh. 1 - Air at 20C with a convection heat transfer...Ch. 1 - Prob. 64PCh. 1 - Prob. 65PCh. 1 - Prob. 66PCh. 1 - Prob. 67PCh. 1 - Prob. 68PCh. 1 - Prob. 69PCh. 1 - Prob. 70PCh. 1 - Prob. 71PCh. 1 - Prob. 72EPCh. 1 - Prob. 73PCh. 1 - Prob. 74PCh. 1 - Prob. 75PCh. 1 - Prob. 76PCh. 1 - Using the conversion factors between W and Btu/h,...Ch. 1 - The outer surface of a spacecraft in space has an...Ch. 1 - Consider a person whose expose surface are is 1.7...Ch. 1 - Prob. 80PCh. 1 - Two surfaces, one highly polished and the other...Ch. 1 - A spherical interplanetary probe with a diameter...Ch. 1 - Prob. 83PCh. 1 - Can all three modes of heat transfer occur...Ch. 1 - Can a medium involve (a) conduction and...Ch. 1 - The deep human body temperature of a healthy...Ch. 1 - We often turn the fan on in summer to help us...Ch. 1 - Prob. 88PCh. 1 - Prob. 89PCh. 1 - Prob. 90PCh. 1 - An electronic package with a surface area of 1 m2...Ch. 1 - Consider steady heat transfer between two large...Ch. 1 - Prob. 93PCh. 1 - Prob. 94PCh. 1 - A 2-in-diameter spherical ball whose surface is...Ch. 1 - Prob. 96PCh. 1 - Prob. 97PCh. 1 - A 3-m-internal-diameter spherical tank made of...Ch. 1 - Prob. 99PCh. 1 - Solar radiation is incident on a 5-m2 solar...Ch. 1 - Prob. 101PCh. 1 - Prob. 102PCh. 1 - Prob. 103EPCh. 1 - An AISI 304 stainless steel sheet is going through...Ch. 1 - Prob. 105PCh. 1 - Prob. 106PCh. 1 - Prob. 107PCh. 1 - Prob. 108CPCh. 1 - Prob. 109PCh. 1 - Prob. 110PCh. 1 - Prob. 111PCh. 1 - Prob. 112PCh. 1 - Prob. 113CPCh. 1 - Why is the metabolic rate of women, in general,...Ch. 1 - What is asymmetric thermal radiation How does it...Ch. 1 - How do (a) draft and (b) cold floor surfaces cause...Ch. 1 - Prob. 117CPCh. 1 - Why is it necessary to ventilate buildings? What...Ch. 1 - Consider a house in Atlanta, Georgia, that is...Ch. 1 - Prob. 120PCh. 1 - A 4m5m6m and room is to be heated by one ton (1000...Ch. 1 - Engine valves (cp=440J/kg.Kandp=7840kg/m3) are to...Ch. 1 - Prob. 123PCh. 1 - Prob. 124PCh. 1 - A 0.3 -cm-thick, 12-cm-high, and 18-cm-long...Ch. 1 - A 40-cm-long, 800-W electric resistance heating...Ch. 1 - It is well known that wind makes the cold air feel...Ch. 1 - An engine block with a surface area measured to be...Ch. 1 - Prob. 129PCh. 1 - Prob. 130PCh. 1 - Prob. 131PCh. 1 - Consider a person standing in a room maintained at...Ch. 1 - Prob. 133PCh. 1 - Prob. 134PCh. 1 - Prob. 135PCh. 1 - Prob. 136PCh. 1 - Prob. 137PCh. 1 - Prob. 138PCh. 1 - Prob. 139PCh. 1 - Prob. 140PCh. 1 - Prob. 141PCh. 1 - Prob. 142PCh. 1 - A 2-kW electric resistance heater submerged in...Ch. 1 - Prob. 144PCh. 1 - A cold bottled drink (m=2.5kg,cp=4200J/kg.K) at...Ch. 1 - Prob. 146PCh. 1 - Air enters a 12-m-long, 7-cm-diameter pipe at 50oC...Ch. 1 - Prob. 148PCh. 1 - Steady heat conduction occurs through a...Ch. 1 - Heat is lost through a brick wall (k=0.72W/m.K),...Ch. 1 - Prob. 151PCh. 1 - A 40-cm-long, 0.4-cm-diameter electric resistance...Ch. 1 - Prob. 153PCh. 1 - Prob. 154PCh. 1 - Over 90 percent of the energy dissipated by an...Ch. 1 - On a still, cleat night, the sky appears to be a...Ch. 1 - Prob. 157PCh. 1 - Prob. 158PCh. 1 - A persons head can be approximated as a...Ch. 1 - A person standing in a room loses heat to the air...Ch. 1 - Write an essay on how microwave ovens work, and...Ch. 1 - Using information form the utility bill for the...Ch. 1 - It is well know that at the same outdoor air...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Explain why on the interval 0<x<1000 mm and 1000<x<2000mm, Mt is equal to positive 160 Nm, but at x= 0mm and x=1000mm Mt is equal to -160 Nm (negative value!). What is the reason for the sign change of Mt?arrow_forward20 3. 2-233 2520 Тр Gears 1079 A pair of helical gears consist of a 20 teeth pinion meshing with a 100 teeth gear. The pinion rotates at Ta 720 r.p.m. The normal pressure angle is 20° while the helix angle is 25°. The face width is 40 mm and the normal module is 4 mm. The pinion as well as gear are made of steel having ultimate strength of 600 MPa and heat treated to a surface hardness of 300 B.H.N. The service factor and factor of safety are 1.5 and 2 respectively. Assume that the velocity factor accounts for the dynamic load and calculate the power transmitting capacity of the gears. [Ans. 8.6 kWarrow_forward4. A single stage helical gear reducer is to receive power from a 1440 r.p.m., 25 kW induction motor. The gear tooth profile is involute full depth with 20° normal pressure angle. The helix angle is 23°, number of teeth on pinion is 20 and the gear ratio is 3. Both the gears are made of steel with allowable beam stress of 90 MPa and hardness 250 B.H.N. (a) Design the gears for 20% overload carrying capacity from standpoint of bending strength and wear, (b) If the incremental dynamic load of 8 kN is estimated in tangential plane, what will be the safe power transmitted by the pair at the same speed?arrow_forward
- Determine the stress in each section of the bar shown in Fig. when subjected to an axial tensile load shown in Fig. The central section is 30 mm hollow square cross- section; the other portions are of circular section, their diameters being indicated What will be the total deformation of the bar? For the bar material E = 210GPa. 20mi О 30mm 30mmm 2.6 15mm 30kN 1 2 10kN - 20kN 3 -329 91mm 100mm 371mmarrow_forwardCalculate the load that will make point A move to the left by 6mm, E=228GPa. The diameters of the rods are as shown in fig. below. 2P- PA 80mm B 200mm 2P 0.9m 1.3m.arrow_forwardIf the rods are made from a square section with the dimension as shown. Calculate the load that will make point A move to the left by 6mm, E=228GPa. 2P- P A 80mm B 200mm 2P 0.9m 1.3marrow_forward
- 3. 9. 10. The centrifugal tension in belts (a) increases power transmitted (b) decreases power transmitted (c) have no effect on the power transmitted (d) increases power transmitted upto a certain speed and then decreases When the belt is stationary, it is subjected to some tension, known as initial tension. The value of this tension is equal to the (a) tension in the tight side of the belt (b) tension in the slack side of the belt (c) sum of the tensions in the tight side and slack side of the belt (d) average tension of the tight side and slack side of the belt The relation between the pitch of the chain (p) and pitch circle diameter of the sprocket (d) is given by 60° (a) p=d sin (c) p=d sin (120° T where T Number of teeth on the sprocket. 90° (b) p=d sin T 180° (d) p=d sin Tarrow_forwardOBJECTIVE TYPE QUESTIONS 1. The maximum fluctuation of energy is the 2. (a) sum of maximum and minimum energies (b) difference between the maximum and minimum energies (c) ratio of the maximum energy and minimum energy (d) ratio of the mean resisting torque to the work done per cycle In a turning moment diagram, the variations of energy above and below the mean resisting torque line is called (a) fluctuation of energy (b) maximum fluctuation of energy (c) coefficient of fluctuation of energy (d) none of the above Chapter 16: Turning Moment Diagrams and Flywheel 611 The ratio of the maximum fluctuation of speed to the mean speed is called 3. (a) fluctuation of speed (c) coefficient of fluctuation of speed 4. (b) maximum fluctuation of speed (a) none of these The ratio of the maximum fluctuation of energy to the.......... is called coefficient of fluctuation of energy. (a) minimum fluctuation of energy (b) work done per cycle The maximum fluctuation of energy in a flywheel is equal to 5.…arrow_forwardOBJECTIVE TYPE QUESTIONS 1. The velocity ratio of two pulleys connected by an open belt or crossed belt is 2. (a) directly proportional to their diameters (b) inversely proportional to their diameters (c) directly proportional to the square of their diameters (d) inversely proportional to the square of their diameters Two pulleys of diameters d, and d, and at distance x apart are connected by means of an open belt drive. The length of the belt is (a)(d+d₁)+2x+ (d₁+d₂)² 4x (b)(d₁-d₂)+2x+ (d₁-d₂)² 4x (c)(d₁+d₂)+ +2x+ (d₁-d₂)² 4x (d)(d-d₂)+2x+ (d₁ +d₂)² 4x 3. In a cone pulley, if the sum of radii of the pulleys on the driving and driven shafts is constant, then (a) open belt drive is recommended (b) cross belt drive is recommended (c) both open belt drive and cross belt drive are recommended (d) the drive is recommended depending upon the torque transmitted Due to slip of the belt, the velocity ratio of the belt drive 4. (a) decreases 5. (b) increases (c) does not change When two pulleys…arrow_forward
- Q3: (10 MARKS) A piston with a weight of 29.4 N is supported by a spring and dashpot. A dashpot of damping coefficient c = 275 N.s/m acts in parallel with the spring of stiffness k = 2400 N/m. A fluctuating pressure p = 960 sin 30t N/m² acts on the piston, whose top surface area is 0.05 m². Determine the steady-state displacement as a function of time and the maximum force transmitted to the base. P=Po sin cot Warrow_forward9. Design a spur gear drive required to transmit 45 kW at a pinion speed of 800 r.p.m. The velocity ratio is 3.5 : 1. The teeth are 20° full-depth involute with 18 teeth on the pinion. Both the pinion and gear are made of steel with a maximum safe static stress of 180 MPa. Assume a safe stress of 40 MPa for the material of the shaft and key. 10. Design a pair of spur gears with stub teeth to transmit 55 kW from a 175 mm pinion running at 2500 r.p.m. to a gear running at 1500 r.p.m. Both the gears are made of steel having B.H.N. 260. Approximate the pitch by means of Lewis equation and then adjust the dimensions to keep within the limits set by the dynamic load and wear equation.arrow_forward7. A motor shaft rotating at 1440 r.p.m. has to transmit 15 kW to a low speed shaft rotating at 500 r.p.m. The teeth are 20° involute with 25 teeth on the pinion. Both the pinion and gear are made of cast iron with a maximum safe stress of 56 MPa. A safe stress of 35 MPa may be taken for the shaft on which the gear is mounted. Design and sketch the spur gear drive to suit the above conditions. The starting torque may be assumed as 1,25 times the running torque. Ruins 20 LW at 100 nm to another shaft running approxiarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Heat Transfer [Conduction, Convection, and Radiation]; Author: Mike Sammartano;https://www.youtube.com/watch?v=kNZi12OV9Xc;License: Standard youtube license