
EBK FOUNDATIONS OF COLLEGE CHEMISTRY
15th Edition
ISBN: 9781118930144
Author: Willard
Publisher: JOHN WILEY+SONS INC.
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 1, Problem 13AE
(a)
Interpretation Introduction
Interpretation:
The number of phases in the image of iodine has to be given.
The given figure is,
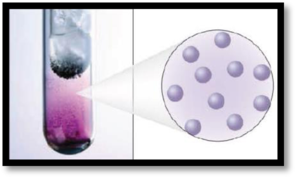
Figure 1
Concept Introduction:
Phase:
Phase is chemically and physically homogeneous quantity of matter that can be separated mechanically from a nonhomogeneous mixture and that may consist of a single substance or a mixture of substances when a phase in one form is altered to another form a phase change is occurred.
(b)
Interpretation Introduction
Interpretation:
The number of phases in the image of bromine has to be given.
The given figure is,
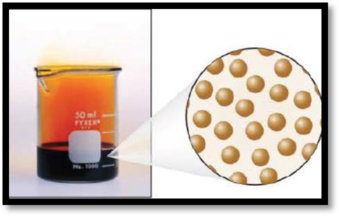
Figure 2
Concept Introduction:
Refer to part (a).
(c)
Interpretation Introduction
Interpretation:
The number of phases in the image of bromine has to be given.
The given figure is,
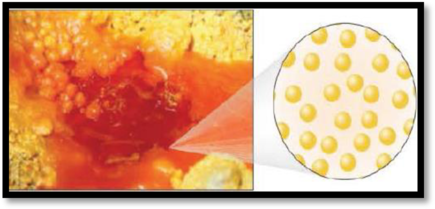
Figure 3
Concept Introduction:
Refer to part (a).
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
You have a solid mixture that contains lithium bromide and barium carbona. Use the Handbook of Chemistry and Physics to propose a method to separate these two solids.
Give an example of a mixture in which solute is solid and solvent is liquid.
Suppose you had a sample of a white crystalline solid that was a mixture of calcium carbonate and calcium chloride. Describe how you could treat the sample to isolate one of the solid in the pure state. Which solid would it be.
Chapter 1 Solutions
EBK FOUNDATIONS OF COLLEGE CHEMISTRY
Ch. 1.1 - Prob. 1.1PCh. 1.4 - Prob. 1.2PCh. 1 - Prob. 1RQCh. 1 - Prob. 2RQCh. 1 - Prob. 3RQCh. 1 - Prob. 4RQCh. 1 - Prob. 5RQCh. 1 - Prob. 6RQCh. 1 - Prob. 7RQCh. 1 - Prob. 8RQ
Ch. 1 - Prob. 9RQCh. 1 - Prob. 10RQCh. 1 - Prob. 11RQCh. 1 - Prob. 12RQCh. 1 - Prob. 13RQCh. 1 - Prob. 14RQCh. 1 - Prob. 15RQCh. 1 - Prob. 16RQCh. 1 - Prob. 1PECh. 1 - Prob. 2PECh. 1 - Prob. 3PECh. 1 - Prob. 4PECh. 1 - Prob. 5PECh. 1 - Prob. 6PECh. 1 - Prob. 7AECh. 1 - Prob. 8AECh. 1 - Prob. 9AECh. 1 - Prob. 10AECh. 1 - Prob. 11AECh. 1 - Prob. 12AECh. 1 - Prob. 13AE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Enter a balanced chemical equation for this reaction. Identify all of the phases.arrow_forwardcopper(II) nitrate and sodium sulfate Express your answer as a chemical equation. Enter NOREACTION if no reaction occurs. Identify all of the phases in your answer.arrow_forward(a) Fe Ss(s) and HBr(aq) Express your answer as a balanced net ionic equation. Identify all of the phases in your answer. ΑΣφ A chemical reaction does not occur for this question. Submit Request Answer Part B K2 CO3(aq) and CuCl2 (aq) Express your answer as a balanced net ionic equation. Identify all of the phases in your answel Write "N.R." if no reaction occurs. ΑΣφ ? A chemical reaction does not occur for this question. Submit Request Answer Part C Fe(NO)(ag) and HCI(ag)arrow_forward
- (a) The sodium ion did not take part in this chemical reaction(Diluted H2SO4(aq) versus NaOH(aq)). What do we call such an ion?(b) Draw a simple diagram which shows how the sodium ion mixes with water in solution.What do we call this physical process?arrow_forwardYou grab a soft drink bottle from your refrigerator. The contents are liquid and remain liquid even when shaken. You now remove the cover, and the liquid solidifies. Provide an explanation for this finding.arrow_forwardWhen calcium oxalate monohydrate is heated, anhydrous calcium oxalate and water are formed. Further decomposition of the calcium oxalate produces calcium carbonate and carbon monoxide. If the heating temperature is increased to well above 600 degrees celsius, calcium carbonate will decompose to calcium oxide and carbon dioxide. Write three reactions to depict the decomposition processess described here.arrow_forward
- Assume that you have two beakers. One is filled with pure water (blue) and the other contains sugar water (green). Which of the following drawings a, b, or c best represents the two beakers after they have been left to evaporate for two days. 2 days ? Pure Water Sugar Water (a) (b) (c)arrow_forwardEnter a balanced chemical equation for the reaction of aqueous potassium hydroxide with aqueous copper(II) chloride to form solid copper(II) hydroxide and aqueous potassium chloride. Express your answer as a chemical equation. Identify all of the phases in your answer.arrow_forward7. Suppose you make a solution that contains 17.59 g of sodium chloride and 960 g of water. (a) What is the mass % of sodium chloride? (b) If 16.47 mL of your solution weighs 16.651 g,what is the density (in g/cc)?arrow_forward
- A sample of hydrated copper (II) sulphate weighs 124.8 g. The sample has been determined to contain 32.8 g of copper (II) ions and 48.0 g of sulphate ions. a) How many molecules of water of crystallization are present in the sample ? b) Deduce the actual formula of hydrated copper (II) sulphate.arrow_forwardOne way the U.S. Environmental Protection Agency (EPA) tests for chloride contaminants in water is by titrating a sample of silver nitrate solution. Any chloride anions in solution will combine with the silver cations to produce bright white silver chloride precipitate. Suppose an EPA chemist tests a 200. mL sample of groundwater known to be contaminated with iron(III) chloride, which would react with silver nitrate solution like this: FeCl3(aq) + 3 AgNO3(aq) 3 AgCl(s) + Fe(NO3),(a9) The chemist adds 56.0 mM silver nitrate solution to the sample until silver chloride stops forming. She then washes, dries, and weighs the precipitate. She finds she has collected 2.8 mg of silver chloride. Calculate the concentration of iron(III) chloride contaminant in the original groundwater sample. Be sure your answer has the correct number of significant digits. mg L Submit Assignment Continue Accessibility Privacy O 2020 McGraw-Hill Education. All Rights Reserved. Terms of Use 888 %23 5 6 2 3 E R. G…arrow_forwardEnter a balanced chemical equation for each of the following. Solid copper reacts with solid sulfur to form solid copper(I) sulfide. Express your answer as a chemical equation. Identify all of the phases in your answer.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY