
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
![In the presence of excess OH", the Zn2+ (aq) ion forms a hydroxide complex ion, Zn(OH)42. Calculate the concentration of free Zn2+ ion when 1.16x10-2 mol Zn(NO3)2(S) is added to
1.00 L of solution in which [OH ] is held constant (buffered at pH 12.10). For Zn(OH)42, K = 4.6x10¹7.
[Zn²+] =
Check & Submit Answer
Show Approach
M](https://content.bartleby.com/qna-images/question/4126b89b-6ee3-437f-8fcf-aa0e90855818/1cd09b40-c201-431b-b1c8-740d19b425ea/4fvwcr_thumbnail.jpeg)
Transcribed Image Text:In the presence of excess OH", the Zn2+ (aq) ion forms a hydroxide complex ion, Zn(OH)42. Calculate the concentration of free Zn2+ ion when 1.16x10-2 mol Zn(NO3)2(S) is added to
1.00 L of solution in which [OH ] is held constant (buffered at pH 12.10). For Zn(OH)42, K = 4.6x10¹7.
[Zn²+] =
Check & Submit Answer
Show Approach
M
Expert Solution
arrow_forward
Step 1
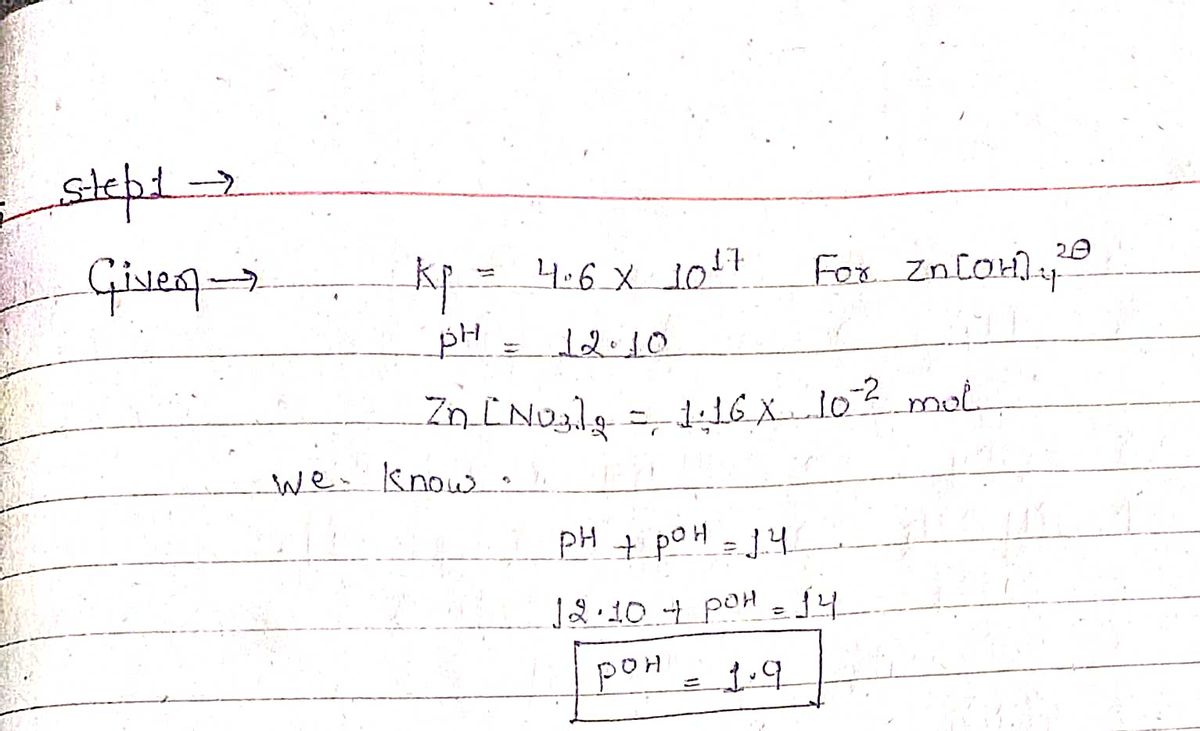
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Rank the compounds in order of increasing symmetry: (a) OF2, (b) CF4, (c) NF3.arrow_forwardThe initial reactive intermediate in the elimination reaction of 2-bromo-3-methyl-pentane is a tertiary carbanion terminal alkene primary carbon radical O tertiary alcohol O secondary carbocationarrow_forward= ||| 오 Identify which of the following structures is most likely to undergo an SN2 reaction. CI CI A) I B) II C) IIIarrow_forward
- Kk.214.arrow_forwardConsider the following molecules. Which of the following statements is correct? OH II OH но.. Question 6 options: 。 I and II are enantiomers 。 о I and III are enantiomers II and IV are diastereomers o III and IV are enantiomers ... III IVarrow_forwardidentify asymmetirc centers with the symbol (*)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY