
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Which of the following arrows or structures contains an error?
Options:
|
|
Arrow 1 |
|
|
Structure 2 |
|
|
Arrow 3 |
|
|
Arrow 4 |
|
|
Structure 5 |
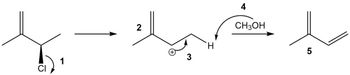
Transcribed Image Text:CI
1
2
3
H
CH3OH
5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 25) Give systematic names for the following compounds. 1. Ca(ClO)2 2. Ag2S2O3 3. NaH2PO4 4. Sn(NO3)2 5. Pb(CH3CO2)4 6. (NH4)2SO4arrow_forwardCircle and label the functional groups in the following:arrow_forwardSelect the correct value for the indicated bond angle in each of the compounds. O-S-O angle of SO₂ 120° <109.5° 180° 90° <120° 109.5° F-O-F angle of OF 2 90° 120° <120° 109.5° 180° O <109.5° F-B-F angle of BF3 180° 90° 120° <109.5° <120° O 109.5° O-C-O angle of CO₂ <109.5° <120° 120° 109.5° 180° 90°arrow_forward
- Can you help me find all of the electron geometry tetrahedral bonds in this Vitimine A structure? The photo attached is one I got wrong. Thank you.arrow_forwardHow would you draw a structure like (+-)hexane-3,4-diol? What about (+)hexane-3,4-diol or (-)hexane-3,4-diol? Would it be impossible to draw these two because + and - separately are determined via polarimetry and not spatial arrangement?arrow_forward1 36 2 37 38 39 A Moving to another question will save this response. Question 9 Classify the reaction shown below. Br x NaOH O rearrangement O substitution O addition O cyclization O elimination xarrow_forward
- 19. Draw the structure for the compound at the bottom of the page. Show your reasoning. C3H1403 100 80 4880 3580 3000 2568 2000 1500 1000 2H 3H 3H 2H 2H 2H 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 PPM 9LC42 86 9 1745.80 BL SS LIC 1486.89 1387. 12 852.85 O 208arrow_forwardDraw the structural formula(s) for the branched constitutional isomer(s) with the molecular formula C-H16 that have two methyl groups on the same carbon. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right comer. • Separate structures with + signs from the drop-down menu. opy aste ChemDoodle"arrow_forwardNonearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY