
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
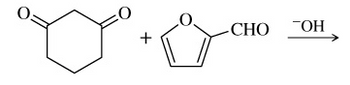
Transcribed Image Text:од
-CHO OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- H3C, CH₂OH H+ А. CH3 OCHS ног СН3 о (1 нохarrow_forwardNaOMe MeoH CH3 Br n-arrow_forwardClassify each of the the following alcohols as primary (1°), secondary (2º), tertiary (3°) using the dropdown on the right. (Hint: Look at the number of carbons [implying alkyl groups] attached to the carbon bonded to the OH group. If only one carbon is attached [that is, 1 carbon and 2 hydrogens], then the alco is primary. If two carbons are attached [that is, 2 carbons and only 1 hydrogen], then the alcoh is secondary. If three carbons are attached [that is, 3 carbons and no hydrogens], then the alco is tertiary.) A B C D OH | CH3-C-CH₂-CH3 | CH3 OH CH3 CH₂ CH₂-CH₂-OH OH Farrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
