
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:www
www
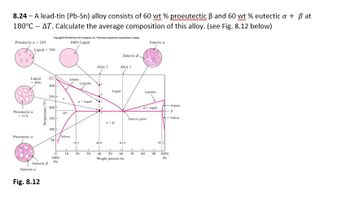
8.24 - A lead-tin (Pb-Sn) alloy consists of 60 wt % proeutectic ẞ and 60 wt % eutectic a + ẞ at
180°C - AT. Calculate the average composition of this alloy. (see Fig. 8.12 below)
Copyright © The McGraw Hil Companies, Inc. Pession required for reproduction or delay
Procutectic a 24%
100% Liquid
Eutectic a
Liquid 76%
Eutectic B
Alloy 2
Alloy 1
Liquid
327
Solidus
= 49%
Liquidas
300
Liquid
Liquidus
250
a + liquid
Procutectic a
-51%
Temperature (C)
200
183
150
Procutectic or
100
18
50
Solvus
19.2
40.0
Solidus
B+ liquid
B
Solvus
Eutectic point
a+B
61.9
97.5
0
10
20
30
40
50
60
70
80
90
100%
100%
Weight percent tin
Sn
Pb
Eutectic B
Eutectic a
Fig. 8.12
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 5. Consider the phase diagram of Cu-Be shown below. At 1000°C with an overall alloy composition of 1 wt% Be, determine the: (a) Phase present, (b) phase compositions, (c) relative amount of each phase, and (d) amount of solid (in grams) that forms in 50 g of the alloy. Include your tie line in the solution. Composition (at% Be) 5 10 15 20 Liquid 1000 7+ 866°C 800 a + Y1 -620°C 600 a + Y2 400 1 4 (Cu) Composition (wt% Be) Temperature (°C)arrow_forwardCopper-rich copper-beryllium alloys are precipitation hardenable. After consulting the portion of the phase diagram shown in Figure below, do the following: Composition (at% Be) 10 15 20 1000 Liquid 866°C 800 -620°C 600 a+ 12 400 (Cu) Composition (wt% Be) (i) Indicate the range of compositions over which these alloys be precipitation hardened. (ii) Describe the heat-treatment procedures (in terms of temperatures) that would be used to precipitation harden an alloy having a composition of your choosing but lying within the range given for part (i). Temper ature ("Carrow_forward20212 -202 A blast furnace makes pig iron containing 3.2% and 1.8% of Carbon and Silicon and remaining as Iron respectively. The ore is a combination of 80%, 12% and 8% of Iron Oxide, Silica and Aluminum Oxide respectively. The process requires coke at a rate of 1 Kg per kg of pig iron produced. The coke is adulterated with Silica with 10% of silica and remaining to be carbon. The flux is obtained at a rate of 0.4 kg per kg of pig iron and is the base of one tonne of pig iron containing pure Calcium carbonate. The blast furnace gas has 30% CO and 15% CO2. calculate the following assuming the 1. The weight of core used. 2. The weight of slag made. m504ssary data required: [15] 504-888/7/2024/04/28-202nsidering t 3. The volume of the blast-furnace gas. 4/28-202123am504-88817-20 Produced,2 88817-2024/04arrow_forward
- 4arrow_forwardONLY LAST SECTION PLEASEarrow_forwardIn a system ABC, a ternary alloy with composition of 30 wt. % B and 30 wt. % C consists at a particular temperature of three phases of equilibrium compositions as follows:Liquid phase: 50 % A, 40 % B, 10 % Calfa solid solution: 85 % A, 10 % B, 5 % Cbeta solid solution: 10 % A, 20 % B, 70 % Ca. Plot the composition of the alloy.b. Calculate the proportion by weight of liquid, and present in this alloy.c. For the same temperature, find the composition of the alloy which will consist of equal proportion of and phases of the compositions stated above, but with no liquid phase.arrow_forward
- A sample of iron ore weighing 800 mg was treated with HNO3 , boiled to dryness and redissolved in dilute HCl. After filtration and removal of undissolved silica, the liquid was passed through a Walden reductor. The collected sample was titrated with 0.0210 M KMnO4 , requiring 12.0 mL to reach the end point. Calc %Fe (55.845) in the ore.arrow_forwardFor the alloy Mg-45 wt% Pb alloy at 500 C, what is the weight percentage of Pb in liquid?arrow_forwardMganga is doing a routine analysis in a copper plating factory. He is regularly making up solutions of verypure CuSO4.5H2O. He suspect that a batch of a reagent used to make up a stock solution of CuSO4.5H2Ois impure and it may contains some CuCl2. He asked you to determine the percentage impurity as follows:Dissolve1.500g of a mixture and make up to 1.00L mark.150mL of this solution was treated with 20.00mLof 0.100M AgNO3 to remove 98.6% of chloride as AgCl precipitate.arrow_forward
- 1. Which of the following compounds can form hydrogen bonds with water?a. octaneb. HClc. NaCld. acetic acid2. Maria wants to determine the percent water content of an unknown hydrate of AB. Given the following data:mass of empty crucible - 12. 2784gmass of AB · xH20 + crucible - 17.4960gmass of AB + crucible - 15.1314gCalculate the % water content of the hydrate3. From the above data, give the correct formula for the hydrate. (AB₂ Molar mass = 129.84 g/mol)arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working!arrow_forwardWhen the high-temperature superconductor yttrium barium copper oxide is heated under flowing H,, the solid remaining at 1000°C is a mixture of Y,O,, BaO, and Cu. The starting material has the formula YBa, Cu,0,-, in which the oxygen stoichiometry varies between 7 and 6.5 (x = 0 to 0.5). %3D 1000°C YBa, Cu, O, (s) + (3.5 – x)H,(g) FM 666. 19-16.00x -Y,0,(s) + 2 BaO(s) + 3 Cu(s)+(3.5 – x)H,O(g) YBa, Cu,Oss Thermogravimetric analysis. When 37.329 mg of YBa, Cu, O, , were subjected to this analysis, 34.435 mg offsolid remained after heating to 1000°C. Find the value of x in YBa, Cu,O, - Propagation of error. Suppose that the uncertainty in each mass is +0.002 mg. Find the uncertainty in the value of x.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY