
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN: 9781938168390
Author: Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Question
Please correct answer and don't use hand rating
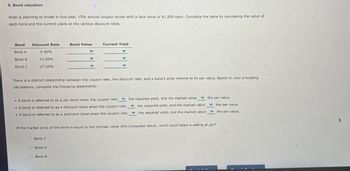
Transcribed Image Text:5. Bond valuation
Roen is planning to invest in five-year, 15% annual coupon bonds with a face value of $1,000 each. Complete the table by calculating the value of
each bond and the current yields at the various discount rates.
Bond
Discount Rate
Bond Value
Current Yield
Bond A
6.00%
Bond B
Bond C
15.00%
17.20%
There is a distinct relationship between the coupon rate, the discount rate, and a bond's price relative to its par value. Based on your preceding
calculations, complete the following statements:
A bond is referred to as a par bond when the coupon rate
A bond is referred to as a discount bond when the coupon rate
A bond is referred to as a premium bond when the coupon rate,
the required yield, and the market value
the required yield, and the market value
the required yield, and the market value
the par value.
the par value.
the par value.
If the market price of the bond is equal to the intrinsic value (the computed value), which bond listed is selling at par?
Bond C
Bond A
Bond B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- You would like to buy a monthly compounding 15-year, $1,000 par value bond. The yield to maturity of this bond is 12 percent. If the annual coupon rate of the bond is an 8.5 percent, what is the value of this bond? Is this bond selling at discount or premium? Group of answer choices $757; Discount $1,225; Premium $1,296; Premiumarrow_forwardBalance the following chemical equation (if necessary):arrow_forwardCombustion reactions take place between a fuel and oxygen and produce carbon dioxide and water. Balance the following combustion reaction C2H2O + CO2 CO2 + H2Oarrow_forward
- Ammonification is the process by which A B C ammonia is released during the decomposition of nitrogen-containing organic compounds ammonium is converted to nitrite and nitrate in soils nitrate from soil is transformed to gaseous nitrogen compounds such as NO, N₂O, and N₂ D gaseous nitrogen is fixed to yield ammonia 4arrow_forwardO Launch Meeting - Zoom O Launch Meeting - Zoom od212ou%3D2591470&isprv &drc3D1&qi=24592388cfql%=D08drnb- ttempt 1 A honeycomb-shape casing of the metals platinum (Pt), rhodium (Rh), and palladium (Pd) in catalytic converters of automobiles cause more reactions to take place that produce less harmful gases without being affected by the reaction. These metals are catalysts because: They cause the reactions without being affected. They are formed into a honeycomb shape. They are metals. They help produce less harmful gasses.arrow_forwardUp [References] Ethanol, the alcohol used in automobile fuels, is produced by the fermentation of sugars present in plants. Corn is often used as the sugar source. The following equation represents the fermentation of glucose, the sugar in corn, by yeast to produce ethanol and carbon dioxide. C6 H12 O6 (aq) → → 2C2H; OH(aq) + 2CO2 (g) Ethanol is combusted in an automobile engine according to the equation C2H; OH(1) + 302(g) → 2CO2 (g) + 3H2O(g) What would be the total volume of CO2 gas formed at STP when 4.45 kg of sugar is fermented and the ethanol is then combusted in an automobile engine? Volume = L Submit Answer Try Another Version 3 item attempts remainingarrow_forward
- Amounts of reactants used and products obtained Molar Mass Volume used Mass used or Moles used or Density (g/mL) (g/mol) (mL) produced (g) produced (mol) 100.158 0.96 Cyclohexanol 1.50 82.143 0.105 Cyclohexene Product theoretical yield (g) Product percent yield (%)arrow_forwardebixoiC 4 During the production of ammonia, hydrogen is produced from the steam reforming of methane. In the reformer methane gas is reacted with excess water and the resulting mixture is found to contain 12 kg of hydrogen and 15 kg water. Assuming all the methane is reacted calculate the amount of water used.arrow_forwardCombustion of hydrocarbons such as pentane (C,H12) produces carbon diaxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon diaxide. 1. Write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. D-0 2. Suppose 0.500 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 19.0 "C. Calculate the volume of carbon dioxide gas that is produced. Round your answer to 3 wignihcant digits.arrow_forward
- 5. Consider the following equation: 2C2H6 + O2 -> 4CO2 + 6H2O When .004 moles of C2H6 are burned the number of moles of CO2 produced will be?arrow_forwardThe psychoactive drug methamphetamine ("speed"), which is sold as the prescription medication Desoxyn, C10H15N, under- goes a series of reactions in the body; the net result of these reac- tions is the oxidation of solid methamphetamine by oxygen gas to produce carbon dioxide gas, liquid water, and an aqueous solution of urea, CH4N₂O. Write the balanced equation for this net reaction.arrow_forward40.0g of KClO3 is subjected to heat until it decomposes entirely. Determine the theoretical yield of Oxygen gas. 2KClO3->2KCl+3O2 1. 51.7g 2. 15.7g 3. 71.5g 4. 15.7garrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div