
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
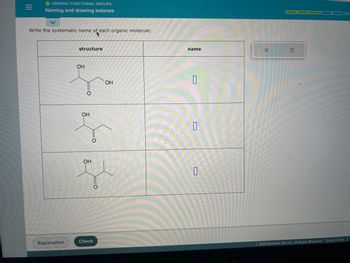
Transcribed Image Text:**Organic Functional Groups: Naming and Drawing Ketones**
**Exercise: Write the systematic name of each organic molecule**
The image contains a table with two columns labeled "structure" and "name". There are three chemical structures drawn in the "structure" column.
1. **First Structure:**
- Contains a ketone group (C=O) with a hydroxy group (OH) attached to the first carbon.
- Appears to be a three-carbon chain with the following substituents: an OH group on the first carbon and the ketone group on the second carbon.
2. **Second Structure:**
- Contains a ketone group with a hydroxy group attached to the second carbon.
- Appears to be a four-carbon chain with the following substituents: an OH group on the second carbon and the ketone group on the carbon next to it.
3. **Third Structure:**
- Contains a ketone group with a hydroxy group attached to the second carbon.
- Appears to be a five-carbon chain with the following substituents: an OH group on the second carbon and the ketone group on the third carbon.
Each structure has an empty box next to it under the "name" column for students to fill in the systematic name.
At the bottom, there are buttons labeled "Explanation" and "Check" to provide feedback or additional resources.
**Note**: The exact systematic names of the molecules need to be filled in by the student based on their understanding of organic chemistry nomenclature.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name the molecule below. (Make sure to type in ALL lower cases for the names of the compounds.) * H,N ОНarrow_forwardName the molecule:arrow_forwardClassify each of these chemical compounds: compound Calla H₂PO4 KNO, type of compound (check all that apply) molecular ionic organic inorganic hydrocarbon molecular ionic organic inorganic hydrocarbon molecular X ionic organic inorganic hydrocarbonarrow_forward
- 4. Write the name for the following compounds a) Your answer b) F CH 3 CH 3 N OH CH 3 1 point ΣΙ 1 pointarrow_forwardRe Part C Interactive 3D display mode ÇH: H2 H;C Spell out the full name of the compound. Submit Request Answer 76F Mostly cloudyarrow_forward7. Name the following compounds: A) CoCl₂ Name: B) P3N5 Name: C) (NH4)3P Name: Name: D) GEO₂arrow_forward
- Classify each of these chemical compounds: compound PCI (CH₂)₂0 C6H6 type of compound (check all that apply) molecular ionic organic inorganic hydrocarbon molecular X ionic organic inorganic hydrocarbon molecular ionic organic inorganic hydrocarbonarrow_forwardWhat is the name of this molecule pictured? НО HO OH OH HO, OH ОНarrow_forwardPlease don't provide handwriting solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY