
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
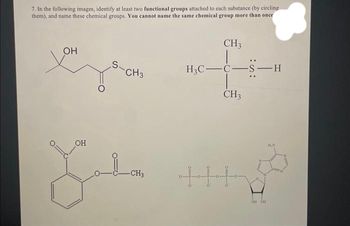
Transcribed Image Text:7. In the following images, identify at least two functional groups attached to each substance (by circling
them), and name these chemical groups. You cannot name the same chemical group more than once
OH
S-CH3
CH3
H3C CS-H
CH3
OH
Leta 113
-CH3
+++
OH OH
1₂N
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ||| NAMING AND DRAWING ORGANIC MOLECULES Interpreting the skeletal structure of a neutral organic molecule Answer the questions in the table below about this molecule: H " 0 What is this molecule's chemical formula? Note: write the simplest molecular chemical formula, in which each element symbol appears only once. How many CH3 CH₂, and CH groups are in this molecule? CH3 CH₂ CH X 3arrow_forwardDraw a 3D formula for the molecule G.arrow_forwardDraw three molecules of each compound below.a. propane, CH 3 CH 2 CH 3b. heptane, CH 3 CH 2 CH 2 CH 2 CH 2 CH 2 CH 3c. 1-propanol, CH 3 CH 2 CH 2 OHd. 1-heptanol, CH 3 CH 2 CH 2 CH 2 CH 2 CH 2 CH 2 OHarrow_forward
- Nonearrow_forward4. Write the name for the following compounds a) Your answer b) F CH 3 CH 3 N OH CH 3 1 point ΣΙ 1 pointarrow_forwardU AAAAAX S1 ▶C Р Vi | MTUTTO TOPTFFFFGo Gob H Question 18 of 19 Provide the correct systematic name for the compound shown here. O H3C iso O di tri cyclo tert- sec- hex benz benz eth ethy hex an yl one MacBook Air 200 2arrow_forward
- Which molecule is a complete organic molecule (i.e. all atoms are shown correctly)? A H. c=C–C= C–H H C H. C=C-C-C-H | H. H. C H. C- D H. нн . C С —С—Н C=C–C- C-H H H. ОН Н А I-0-I エーO I-Ú-I I-U-I I-U-I エー○arrow_forward||| NAMING AND DRAWING ORGANIC MOLECULES Interpreting the skeletal structure of a neutral organic molecule Answer the questions in the table below about this molecule: H Y What is this molecule's chemical formula? Note: write the simplest molecular chemical formula, in which each element symbol appears only once. How many CH3, CH₂, and CH groups are in this molecule? 4 1 CH3 CH₂ CH 00 X - 3/5 Śarrow_forwardWhich statement best describes the bond length(s) in the nitrite ion (shown)? The bond length of N-O is 136 pm and the bond length of N=O is 122 pm. Y-M |-- A. Both bond lengths are 258 pm. B. There are two bonds lengths, one is 136 pm and the other is 122 pm. C. Both bond lengths are 129 pm. O D. Both bond lengths are 136 pm.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY