
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
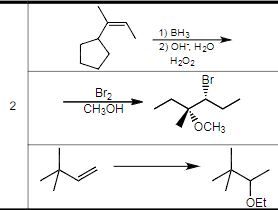
Transcribed Image Text:2
Br₂
CH OH
1) BH
2) ОН: Н20
H202
OCH3
OEt
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ||| A ALEKS-Katelyn Pearson... 1 Apromatincat Rom Fecr] ATOMS, IONS AND MOLECULES Predicting the formula of ionic compounds with common... Write the empirical formula for at least four ionic compounds that could be formed from the following ions: OH, Fe²+, 103, NH b Answered. The following X O Garrow_forwardThe chemical structure of lactic acid (C,H,03) is shown below. Highlight each atom that is in a hydroxy group. H :0: ? Н —О C C -0. - - .. Н —с — Н H :0 :arrow_forward2 HC= CH + 5 O2 > 4 co2 + 2 h2o What is the energy of the reaction and is it exo- or endothermic?arrow_forward
- (b) 0.36 M NaHCO3 lons: O3 Na* NaH* O CO;²- O NaHC* O HCO3 [cation*] = i M [anion] = Marrow_forward(4b-101) Only using the list of ions below, form two ionic compounds that would be soluble in water, and two ionic compounds that would be insoluble in water. You should make four different compounds, but you may use ions more than once. Be sure to indicate which is which, and make sure your compounds are charge-balanced! Na+ Cour... Ca2+ Ni3+ NH4* s2- so42- Br OH For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac). BIUS Paragraph Arial 14px A v Ix E = = E x² X2 次 T T ABC 田 田田图 - (;} Click Save and Submit to save and submit. Click Save All Answers to save all answers. Save All Answers Save and Submit APR 1600 4,104 Aa MacBook Air 80 000 O00 F1 F2 DII DD F3 F4 F5 F6 F7 F8 F9 F10 F11 F12 @ 23 $ & ( ) 1 2 3 4 5 6 7 8 %3D delete Q W E R T Y { } 121 II 了E 田 * +] + 21arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY