
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
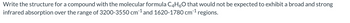
Transcribed Image Text:Write the structure for a compound with the molecular formula C4H6O that would not be expected to exhibit a broad and strong
infrared absorption over the range of 3200-3550 cm ¹ and 1620-1780 cm ¹ regions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Molecular Recognition (Supramolecular Chemistry) in organic chemistry deals with the "lock and key " mechanisms that form new molecules. However, little is known of this subject when moleules go beyond 1500 daltons (g/mole) for example macromolecules of polyolefins (polyethylene, polypropylene), thermoplastic polyesters and polyamides (nylon). It is understood that pre-directional H bonding is the mechanism of molecular recognition of macromolecules. Question: What is the immediate and post manifestation of inducing molecular recognition to condensation polymers and ring opening polymerization polymers like thermoplastic polyesters (PET ), and Nylon 66, 6 etc. (polyamides)?arrow_forwardIn infrared spectra, the O-H vibrational frequency of alcohols occurs as a broad peak (peak absorbable) in the region 3200-3600 cm -1, true or false?arrow_forward7. Consider the C=O stretching data for the following cyclic ketones: cyclopropane 1813 cm³¹; cyclobutanone 1791 cm³¹; cyclopentanone 1740 cm³¹. (a) which ketone has the strongest bond? (b) where in the trend would you expect the C=O stretch of cyclopropenone? (Use the hybridization information in the same way we used hybridization for C-H stretching.)arrow_forward
- Identify and explain the IR absorption characteristics of the unknown C6H14O in the 1400 to 4000 cm-1 range. Then identify the class of the unknown compound C6H14O. At the broad peak I believe it is either an alcohol & phenol or an amine. For the second peak i think it is an alkane.arrow_forwardWhich of the following molecules would be expected to have absorbance in the IR range from a symmetric stretching vibration? SO2 CS2 CF4 CO2 CH4arrow_forwardhelparrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY