
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
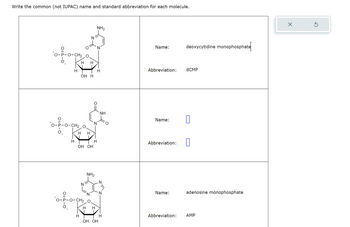
Transcribed Image Text:Write the common (not IUPAC) name and standard abbreviation for each molecule.
O-P-O-CH20
H H
NH2
Name:
deoxycytidine monophosphate
H
H
Abbreviation:
dCMP
OH H
O-P-O-CH2 0
H H
H
OH OH
NH2
NH
Name:
П
H
Abbreviation:
☐
CH2 O.
H H
H
OH OH
Name:
adenosine monophosphate
H
Abbreviation:
AMP
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Xylulose has the following structural formula. To what carbohydrate class does xylulose belong based on the number of carbons and carbonyl functionality? A) aldotetrose B) aldopentose C) ketotetrose D) ketopentose E) ketohexosearrow_forwardClassify the following monosaccharides as an aldose or ketose. I need help on number 5, a-d.arrow_forwardWhat kind of saccharides are these?arrow_forward
- i H₂N-CH-C-N-CH-C-N-CH-C-OH CH₂ i CHCH₂ CH₂ H H H + water HCI valine phenylalanine + glycinearrow_forwardWhich of the following describes the following monosaccharide: CH;OH H- OH HO- CH,OH aldose and pentose ketose and pentose aldose and tetrose O ketose and tetrose O aldose and hexose O ketose and hexosearrow_forwardFill in the empty blanks and spaces in the table. The structure of d-galactose is in the next picture for reference.arrow_forward
- Convert these in Haworth forms and show alpha and beta formsarrow_forwardFor each of the following disaccharides, circle the glycosidic linkage and indicate the type of glycosidic linkage:arrow_forwardWhich of the following is the correct pair of monosaccharides needed 2 to form the given glycoside? * CH2OH CH-OH CH2OH OH он он OH OH a-D-fructofuranose & B-D-glucopyranose a-D-sorbofuranose & B-D-allopyranose a-D-mannofuranose & B-D-glucopyranose O a-D-sorbofuranose & B-D-galactopyranosearrow_forward
- D The following disaccharide contains a(n) (blank]-glycosidic linkage. HO CH₂OH ОН Oa-1,4 OB-1,4 a-1,6 O B-1,6 0 OH Question 6 double bond ssignments/syllabus ester bond ether bond amide bond HO A glycosidic bond between two monosaccharides can also be classified as a(n) [blank). OH OH Melebi OH MacBook Proarrow_forwardIdentify the type of glycosidic bond in the following disaccharide. CH,OH H H ОН Н CH2 H H H ОН Н HO ОН H. ОН HO H H OH O B-1,4-glycosidic bond O a-1,4-glycosidic bond O B-1,6-glycosidic bond O a-1,6-glycosidic bondarrow_forwardN-NHPH CHO CH2OH H- C=N-NHPH Но- -H- 3 equiv Но- -H- 3 equiv Но- PHNHNH2 PHNHNH, H- H- OH H- -O- H- H- -HO- H- -HO- ČH2OH ČH2OH ČH2OH D-glucose osazone D-fructose + NH3 + PHNH2 + 2 H20 The final step in the formation of the osazone from glucose is the reaction of the keto imine with 2 equivalents of phenylhydrazine to yield the osazone plus ammonia. This reaction involves the following intermediate steps: 1. Addition of phenylhydrazine to the imine and proton transfer to yield intermediate 1; 2. Elimination of ammonia to yield phenylhydrazone 2; 3. Addition of phenylhydrazine to the ketone to yield tetrahedral intermediate 3; 4. Proton transfer yields carbinolamine 4; 5. Elimination of water yields the final product osazone. Write out the mechanism on a separate sh of paper and then draw the structure of tetrahedral intermediate 3. NH НО- -H H- -ОН H- -ОН ОН Glucose keto imine Previous Nearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning