
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please don't provide handwritten solution ...
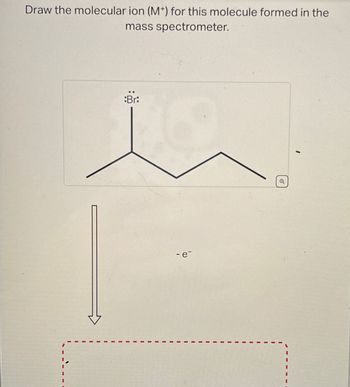
Transcribed Image Text:Draw the molecular ion (M+) for this molecule formed in the
mass spectrometer.
Br:
- e-
6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Which of the following formulae is consistent with a molecular ion of m/z 73 in a mass spectrometry experiment? O C3H8N2 O C4H11N O C4H10O O C3H5CIarrow_forwardDraw the molecular ion (M+) for this molecule formed in the mass spectrometer. I - e- Drawing :0: ОН Qarrow_forwardnot use ai pleasearrow_forward
- Match the molecular ion (from electron impact mass spectrometry) with the correct compound. (Make sure to consider the isotope pattern) 100 120 Br A 121 x B NH ន 122 Mass M/e 123 123 Me 124 124 OH но ма 125 D 126arrow_forwardUse the isotope method to determine the molecular formulaarrow_forwardIn the mass spectrum of an unknown organic compound, the molecular ion had a relative abundance of 19.0 while the M+1 had a relative abundance of 1.5. Estimate the number of carbons in the molecule. 1 4 7 2 LO 5 8 carbons 3 6 9 X Carrow_forward
- In a mass spectrum, what m/z value would correspond to the fragment shown below?arrow_forwardUsing the rule of 13, what is a possible formula for M=113 (100%) on a mass spectrum? all of the above are possible molecular formulas CH130 CH15Narrow_forwardplease help identify the mass spectrometryarrow_forward
- Halogenated compounds are particularly easy to identify by their mass spectra because both chlorine and bromine occur naturally as mixtures of two abundant isotopes. Recall that chlorine occurs as 35Cl (75.8%) and 37Cl (24.2%); and bromine occurs as 79Br (50.7%) and 81Br (49.3%). At what masses do the molecular ions occur for the following formulas? What are the relative percentages of each molecular ion? (a) Bromomethane, CH3Br (b) 1-Chlorohexane, C6H13Clarrow_forwardDraw the molecular ion (M*) for this molecule formed in the mass spectrometer. H. H -e-arrow_forwardDetermine if the compounds contain a Chlorine, Bromine or Fluorine atom.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning