
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
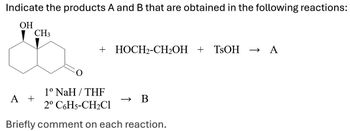
Transcribed Image Text:Indicate the products A and B that are obtained in the following reactions:
OH
CH3
+ HOCH2-CH₂OH + TSOH → A
1° NaH/THF
A +
→>
B
2º C6H5-CH2Cl
Briefly comment on each reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Draw the structural formula for the major product of the following reactions.arrow_forwardAn important step in one synthesis of carboxylic acids is the deprotonation of diethyl malonate and its alkyl-substituted derivative: Base CH;CH2O OCH,CH3 CH;CH,0 OCH2CH3 H2 Diethyl malonate Base CH;CH,0 °C `OCH,CH3 CH;CH,O OCH,CH3 R Alkyl substituted diethyl malonate NaOH can deprotonate diethyl malonate effectively, but NaOC(CH3)3 is typically used to deprotonate the alkyl-substituted derivative. Explain why.arrow_forwardWhich compound is the dominant product in the reaction shown below? KMNO4 .COOH (A) H*/H,O (B) COOH .COOH (C) (D) HOOC HOOC Compound B Compound C Compound D Compound Aarrow_forward
- Nonconjugated , -unsaturated ketones, such as 3-cyclohexenone, are in an acid-catalyzed equilibrium with their conjugated , -unsaturated isomers. Propose a mechanism for this isomerization.arrow_forwardArrange these compounds in order of increasing acidity: 2,4-dichlorophenol, phenol, cyclohexanol.arrow_forwardThe key step in a reported laboratory synthesis of sativene, a hydrocarbon isolated from the mold Helminthosporium sativum, involves the following base treatment of a keto tosylate. What kind of reaction is occurring? How would you complete the synthesis?arrow_forward
- Bhu Please don't provide handwritten solution Write the mechanism of the reaction show with proper arrow formations. Do not combine in one steparrow_forwardProvide synthetic routes for the preparation of the starting materials of azo dye Alizarine Yellow R starting from benzene O₂N -NEN OH CO₂Harrow_forwardProvide the major products of the given reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning