
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
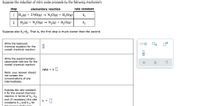
Transcribed Image Text:Suppose the reduction of nitric oxide proceeds by the following mechanism:
step
elementary reaction
rate constant
1 H,(g) + 2 NO(g) → N20(g) + H,O(g)
k1
2
H2(g) + N,0(g) →
N,(g) + H,O(g)
k2
Suppose also k,«k,. That is, the first step is much slower than the second.
Write the balanced
chemical equation for the
overall chemical reaction:
Write the experimentally-
observable rate law for the
overall chemical reaction.
rate = k||
Note: your answer should
not contain the
concentrations of any
intermediates.
Express the rate constant
k for the overall chemical
reaction in terms of k, k2,
and (if necessary) the rate
constants k.1 and k.2 for
the reverse of the tae
k =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The data given in the table below is for the following reaction (run at 25°C): 2 NO + O, 2(g) → 2 (g) 2(g) Initial Concentration (M) Initial rate Experiment NO 0, Mol/(L · s) 1 0.0010 0.0010 7 x 10-6 2 0.0010 0.0020 14 x 106 3 0.0010 0.0030 21 x 10-6 4 0.0020 0.0030 84 x 10-6 5 0.0030 0.0030 189 x 106 1. Determine the orders of the reaction with respect to NO and O, (show all work): NOarrow_forwardSome measurements of the initial rate of a certain reaction are given in the table below. N2 H, initial rate of reaction 1.74 M 2.29M 0.220 M/s 1.74 M 0.883 M 0.0327 M/s 0.788 M 2.29 M 0.0451 M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. rate = k || Ox10 ロロ ロロ k =arrow_forwardDetermine the rate law and the rate constant for this reaction. 13.125. The table contains reaction rate data for the reaction 2 NO(g) + Cl,(g)→ 2 NOCI(g) Initial Rate Experiment [NO], (M) [Cl_]o (M) (M/s) 1 0.20 0.10 0.63 0.20 О.30 5.70 0.80 0.10 2.58 0.40 0.20 Predict the initial rate of reaction in experiment 4. 13.126. An important reaction in the formation of photochemical smog is the reaction between ozone and NO: NO(g) + O3(g) → NO;(g) + O,(g) 2. 2. 3.arrow_forward
- estimate the value of the rate constant karrow_forwardO KINETICS AND EQUILIBRIUM Deducing a rate law from initial reaction rate data Some measurements of the initial rate of a certain reaction are given in the table below. N2 H, initial rate of reaction 2.21 M 2.33 M 35.0 M/s 2.21 M 4.98M 160. M/s 7.27 M 2.33 M 379. M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. rate = k O k = | Explanation Check O 2021 McGraw-Hill Educ P Type here to search IIIarrow_forwardGiven the information below, determine the rate law for the reaction and the rate constant (k). Be sure to include the units for the rate constant (k). A + 2B --> C + D [A] M [B] M Initial Rate (M/s) 0.10 0.10 0.0015 0.20 0.10 0.0060 0.20 0.20 0.0120 0.40 0.10 0.0240 rate law: rate constant k:arrow_forward
- During a chemical reaction the temperature drops from 26 oC to 14 oC. What can you conclude about the reaction? It has a high activation energy It is a combination reaction It is an endothermic reaction It is an exothermic reactionarrow_forwardThe rate law for the reaction 2NO(g) + 2H₂(g) N₂(g) + 2H₂O(g) is rate = K[NO]²[H₂] -1 If the rate of reaction is 4.66 × 10-3 mol L-¹s¹ when the concentrations of NO and H₂ are both 6.9 x 10-6 mol L-¹, what is the value of the rate constant and what are the units for the rate constant? k= i x 1013 X 9.56 5-1 mol L-¹5-1 L³ mol-³ s-1 3 L² mol-2 s-1 L mol-¹ S-1arrow_forwardSome measurements of the initial rate of a certain reaction are given in the table below. N2|||H2|initial rate of reaction 2.46M 2.06M 0.603M/s 11.3М| 2.06М 12.7M/s 2.46M 5.01М 3.57M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. rate = k ||| k =arrow_forward
- Some measurements of the initial rate of a certain reaction are given in the table below. [₂] [H₂] initial rate of reaction 0.619M 0.967 M 2.00 × 105 M/s 0.619M 3.31M 2.97M 0.967 M Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 2 significant digits. Also be sure your answer has the correct unit symbol. rate = k 2.34 × 10 M/s 9.60 × 105 M/s k = 0 0 x10 ロ・ロ Xarrow_forwardConsider the reaction 2HI→ H₂+I2. The rate of disappearance of HI follows the second order rate law. A[HI] = -k[HI]² At The rate constant kis 2.01 x 10-2 M-1s-1. What was the initial concentration of HI (in units of molarity) if the concentration of HI was found to be 0.262 M after t = 45.0 s? Report your answer using three significant figures. Provide your answer below: M ratearrow_forwardThe rate constant k for a certain reaction is measured at two different temperatures: temperature k 142.0 °C 6.1 x 1011 226.0 °C 3.7 x 10¹2 Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy E for this reaction. Round your answer to 2 significant digits. mol ×arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY