
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
With the help of the half reactions given in Table 1, formulate the redox equation for the oxidation of succinate and reduction of ubiquinone.
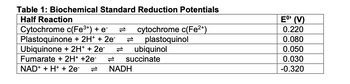
Transcribed Image Text:Table 1: Biochemical Standard Reduction Potentials
Half Reaction
Cytochrome c(Fe³+) + e¯
Plastoquinone + 2H+ + 2e-
Ubiquinone + 2H+ + 2e-
Fumarate + 2H+ +2e-
NAD+ + H+ + 2e-
cytochrome c(Fe²+)
plastoquinol
NADH
ubiquinol
succinate
EO (V)
0.220
0.080
0.050
0.030
-0.320
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Similar questions
- 1. In a catabolic pathway, metabolite X gets converted into metabolite Y, metabolite X 0 J mol-¹ and AG' is <0 J mol-¹ for this reaction. a) At standard states, is this reaction favorable? b) Is the reaction favorable or unfavorable at cellular condition? c) Is this an endergonic or exergonic reaction in the cell? d) Would you describe this as a thermodynamically downhill or uphill reaction in the cell?arrow_forwardAnswer a to d for amine reactionarrow_forwardWrite out the full redox reaction that happens when both of these reactions are added to the same reaction where there is 1M concentrations of each reactant and products, pH7, and temeprature is 290K. Identify which compounds will be reduced and which compounds will be oxidized. redox reaction 1: Pyruvate¯ + 2H+ + 2e¯ → lactate¯ E'o(V) = -0.185 redox reaction 2: NAD+ + H+ + 2e¯ → NADH E'o(V) = -0.320arrow_forward
- Under standard conditions, will the following reactions proceed spontaneously as written? (1) Fumarate + NADH + H+ (2) succinate + NAD+ Cyto a (Fe²+) + cyto b (Fe³+) = cyto a (Fe³+) + cyto 6 (Fe²+) barrow_forwardConsider the reaction catalyzed by PFK. In the presence of AMP, which of the following will be expected? Check all that apply: a)The Km for substrate would be decreased b) the initial velocity plot would show the curve shifted to the right c) the R state is stabilized d)the rate of the reaction is diminishedarrow_forwardWhat would be the characteristics of a transitionstate analog for the chymotrypsin reaction?arrow_forward
- Explain IN DETAIL the process of Kreb’s Cycle. Include the overall equation, location, products, by-products, and reactants.arrow_forwardSelect the following enzymes that utilize a mechanism where an enediol intermediate is formed. Check all that apply: a)phosphoglucoisomerase b)triose phosphate isomerase c)aldolase d) glyceraldehyde 3-P dehydrogenase e) hexokinasearrow_forwardi) Re-arrange the Michaelis Menten equation so it involves the ratio [S]. Show all steps beginning Km noting any assumptions or required conditions. Km ii) Calculate the ratio [lo for the case when the rate of product formation is 68% of Vmax and the substrate is in great excess. d[P] dt : k₂ with = [E],[S] Km+[S]' [S]o Km iii) Explain, in a few sentences, why the ratio determines the ratio V Vmax V Vmax Begin by explaining the meaning of stating simply "it's the ratio...." is not sufficient. Include in your explanation the factors that effect v and Vmax. Consider what factors make v different from or equal to Vmax. Consider what Km represents concerning processes involving ES. " iv) Calculate KM at 310K at given the following rate constant information: k₁ = 17 s-¹M-1 at 300K with A = 7300 s-¹M-1 K-1₁ 6 s¹ at 300K with A = 14500 s -1 k₂ = 31 s¹ at 300K with A = 600 s-¹arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON