
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
why is the T at P=5Mpa is equal to 200 deg C?

Transcribed Image Text:1. Using the Steam Tables, determine heat absorbed by the boiler (KJ) when subcooled water at pressure = 5 Mpaa (subcooled by 64 deg
C measured at the boiler inlet), is superheated to 100 degrees superheat at the same pressure. Total volume of H2O heated is at 10 +
SN/10 cubic meters. Draw also the PV and TS diagrams below with labels/ identification of all lines or curves. Answer: Qa =.
т
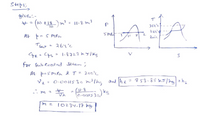
Transcribed Image Text:given'.-
+ = (10 t18_) m? - 11-8 m3
364°c
264°
2.
Tsat
264°C
pe- Cps= 1.8723 kJ/Kg
for sub loaleed Steam ;
At þesmfa &T= 200°%
Ve: O:v0il5 30m3lkg =h
and he
853.85 kJ/ hg
,^ m 2
(1.8
kg
10234.17 kg
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- consider the mass and pulley system in the attached file. mass m1 = 29 kg and mass m2 = 12kg. the angle of the inclined plane is given and the coefficient of kinetic friction between mass m2 and the inclined plane is uk = 0.12. assume the pulleys are massless and frictionless when mass m2 moves a distance 4.94 m up the ramp, how far downward does mass m1 move? d= ?arrow_forwardA vessel of water containing 300 kg of water is initially at 270 K. A 100 kg piece of metal,initially at 1300 K, is placed in the water vessel to cool and the vessel is closed. The specificheat of the metal is 0.6 kJ/kg K and 4.2 kJ/kg K for water. Assume the specific heats areconstant and that the vessel is well insulated.a. Find the final equilibrium temperature after cooling. b. Find the amount of entropy generated within the vessel in kJ/K.arrow_forwardA fixed volume of propane is initially at T = 420 K and P = 3 MPa. The temperature is raised to T = 500K. Find the current pressure of the propane with thermodynamic reasoningarrow_forward
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY