
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
The technician then plates 0.1mL of the last three dilutions in duplicate. Why are the last three dilutions plated, as opposed to only one dilution? Why are the dilutions plated in duplicate?
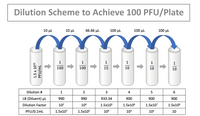
Transcribed Image Text:Dilution Scheme to Achieve 100 PFU/Plate
10 μιL
10 μι
66.66 µl
100 μL
100 μL
100 μι
1
1
1
1
1
1
100
100
15
10
10
10
Dilution #
1
3
4
6.
LB (Diluent) µl
990
990
933.34
900
900
900
Dilution Factor
102
104
1.5x105
1.5x106
1.5x107
1.5x108
PFU/0.1mL
1.5x107
1.5x105
104
103
102
10
1.5 x 1010
PFU/mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The market supply of lettuce in a small town is shown in the table below. Supply of Lettuce Price (dollars per Quantity of Lettuce Supplied (heads) head) Initial New $4.00 400 3.50 300 3.00 200 2.50 100 Instructions: Enter your answers as a whole number. a. Suppose an increase in the cost of water decreases production of lettuce heads by 30% at every price. Complete the new supply schedule in the table above. b. At a price of $3.50 per head of lettuce, the original quantity supplied was heads of lettuce and the new quantity supplied is heads of lettuce.arrow_forwardStarting from the primary standard solution in Question 4 and using the dilution scheme in Figure 1, calculate the concentrations of Standards #1-5. Show all work on a piece of scratch paper. Label each calculation clearly (Standard #1, Standard #2, etc) and circle your final answer for each standard. Be sure to round your answers to the correct number of significant figures and include units. Molarity in #4 is 0.0004089 M or 4.089x10^-4arrow_forwardthis question was rejected because the person said it appeared incomplete but this is the full question !arrow_forward
- Based off information from the attachment, please help with #9 on second attachmentarrow_forwardA student pipetted 10 mL of a sample solution into a 250 mL volumetric flask and diluted it to the mark with distilled water. The student then, pipetted 10 mL of this new solution into a 50.00 mL volumetric flask and diluted it to the mark with water. What is the dilution factor of the final solution? 25X 100X 125X 5X 50Xarrow_forward5.00 mL of stock solution is diluted to 25.00 mL, producing solution ALPHA. 10.00 mL of solution ALPHA is diluted to 25.00 mL, resulting in solution BETA. 10.00 mL of solution BETA is then diluted to 25.00 mL, producing solution GAMMA. dilution factor for ALPHA from stock solution = 0.167 dilution factor for BETA from ALPHA solution = 0.0476 part c and d?arrow_forward
- Data obtained: Chips # of extractions Chips' weight (g) Fat weight (g) Regular 3 20.043 6.745 3 20.187 6.438 3 20.198 7.451 Low fat 3 19.456 3.982 3 20.072 4.547 3 20.192 4.589 Mass percent is a method of expressing the concentration of a substance in a mixture or element in a compound. It is calculated as the mass of the component divided by the total mass of the mixture and then multiplied by 100 to get the percent. The formula is: mass % of component= (mass of component / total mass) x 100% 1) Reproduce the following table, and use the data to determine the mass % of fat in the chips for each trial. 2) Show one example calculation. Chips Trial % by mass of fat Regular 1 2 3 Lowfat 1 2 3arrow_forwardEpsom salt is a product that may be added to a bath to help ease sore muscles. Epsom salt crystals Epsom salt ground crystals A materials scientist tested two forms of Epsom salt under different conditions to see which would dissolve the fastest. The test information is displayed in this table. Sample Mass Form Water temperature A 50 grams ground crystals 30°C B 100 grams crystals 20°C 50 grams 100 grams ground crystals 20°C crystals 30°C Which sample of Epsom salt would you expect to dissolve the fastest? O A. A В. В ос. С O D. Darrow_forward2.00 mL OF 5.80 x10-"M AGNO g 245.0 mL of 3.35 X10 M NazS and + Na, Scaas 2 NANO3 tazy 248.19 1549 2709 2 Ag NO3 78.19 Nans Agzs 2 NANO3 inirial Change Finalarrow_forward
- 1arrow_forwardQ) A student misreads the directions and adds 4.0 g of benzoic acid instead of 0.4 g. What do you expect to happen? Would the experiment still be valid? Explain your reasoning. Pure Lauric Acid Lauric Acid + 0.4 g Benzoic Acid Lauric Acid + 0.5 g Benzoic Acid Mass of Lauric Acid 3.03g 3.00g 3.00g Mass of Benzoic Acid 0 g 0.40g 0.50g Freezing Point 43’C 39’C 38’C Freezing Point Depression * 4’C 5’C Molality of Benzoic Acid in Lauric Acid* 1.026 mol/kg 1.282 mol/kg Moles of Benzoic Acid * 0.003 moles 0.004 moles Experimental Molar Mass of Benzoic Acid * 133.3g/mol 125 g/mol Average Molar Mass * 129.15g/mol Percent Error * 5.75%arrow_forwardAn impure sample contains 1.01 g of impurities and 4.92 g of benzoic acid. The impure sample is dissolved in water, then heated. The solution is then cooled. How many grams of benzoic acid will recrystallize when the solution has cooled? Include the unit and two decimal places in your answer. The solubilities of the impurity and benzoic acid in hot water are found as an item in the Recrystallization Experiment folder. Solubility Data for Report Questions 10 Compound Cold Water solubility Hot Water Solubility Benzoic acid 0.42 g per 100 mL of water 5.79 g per 100 mL of water Impurity 0.61 g per 100 mL of water 4.62 g per 100 mL of waterarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY