
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
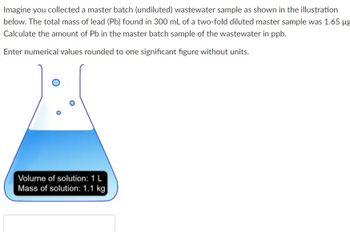
Transcribed Image Text:Imagine you collected a master batch (undiluted) wastewater sample as shown in the illustration
below. The total mass of lead (Pb) found in 300 mL of a two-fold diluted master sample was 1.65 µg
Calculate the amount of Pb in the master batch sample of the wastewater in ppb.
Enter numerical values rounded to one significant figure without units.
O
Volume of solution: 1 L
Mass of solution: 1.1 kg
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the molarity of a solution in which 50 g of NaOH (molar mass 40.00 g/mol) is dissolved in 1500. mL of solution? 0.033 M O 0.026 M 0.833 M 33.3 M MacBook Air 20 000 esc 000 F4 F8 F10 F6 F7 F9 F1 F2 @ 2$ & * %3D 4 5 6 7 8 W E R T Y U %# Marrow_forwardAn analytical chemist weighs out 0.138 g of an unknown monoprotic acid into a 250 mL volumetric flask and dilutes to the mark with distilled water. He then titrates this solution with 0.0600M NaOH solution. When the titration reaches the equivalence point, the chemist finds he has added 25.5 mL of NaOH solution. Calculate the molar mass of the unknown acid. Round your answer to 3 significant digits. 0 mol X S 629 OL Aarrow_forwardPart 1,2and3arrow_forward
- Please don't provide handwritten solution ...arrow_forwardPlease help me answer questions 1 and 2. (Questions attached below) 1. When you arrive at the lab, 2.0 M H2SO4(aq) and 2.0 M HC2H3O2(aq) will be present. Youwill need to dilute these solutions using beakers and graduated cylinders from your drawerand DI water.i. Calculate the volume of the 2.0 M HC2H3O2(aq) and DI water needed to create 50.0 mLof 1.05 M HC2H3O2(aq).ii. Calculate the volume of the 2.0 M H2SO4(aq) and DI water needed to create 50.0 mL of0.75 M H2SO4(aq). 2. A 48.33-mL sample of 0.150 M NaBr is titrated into 50.00 mL of 0.145 M AgNO3. Bothsolutions had the same initial and final temperatures. The reaction occurred in a calorimeterwith a known heat capacity and produced 1546.03 J of heat.i. Write a balanced equation that is occurring.ii. Determine the theoretical yield of the solid.iii. Calculate the enthalpy of the reaction (kJ/mole of precipitate).arrow_forwardA student prepared a standard copper solution by combining 2.63 mL of stock 0.270 mol/L copper(II) sulfate solution with 6.37 mL of water. What is the molar concentration of Cu2+ in this solution? Give your answer in in decimal format. When entering units, use proper abbreviated units with proper capitalization.arrow_forward
- An analytical chemist weighs out 0.087 g of an unknown monoprotic acid into a 250 mL volumetric flask and dilutes to the mark with distilled water. She then titrates this solution with 0.1700 M NaOH solution. When the titration reaches the equivalence point, the chemist finds she has added 5.4 mL of NaOH solution. Calculate the molar mass of the unknown acid. Round your answer to 2 significant digits.arrow_forwardA solution is prepared by dissolving 72.91 g of CdCl2 (MM = 183.317) in enough water to make 158.95 mL of solution. If the density of the solution is 1.556 g/mL, what is the molarity of the chloride ions, Cl-(aq), in solution? Report your answer using two decimal places.arrow_forwardPlease help me with this one Sugar is the solute and water is the solvent. The drink mix will be considered negligible and is added simply for the visualization of dilution results. Calculate the concentration of sugar in this solution using the volume percent equation. Refer to the background for sample calculations. For the purpose of this exercise, it can be assumed that the weight of the drink mix is 0.00 g. The volume of the solution is 3 and 1/3 cups. Convert to metric units, then calculate and record the volume percent in Data Table 1 (Note that 1 cup = 236 mL). Volume % = Volume of Solute/ Volume of Solution × 100%arrow_forward
- Most analytical machines used in science can either measure absorbance of light, like the spectrophotometers used BioZ 151 and 152 labs, or can measure change in electrical conductance. In order to convert things like absorbance values into concentration data, a linear regression is used. In order to determine nitrite concentration in water samples, nitrite is reacted with several chemicals to produce a purple color. The absorbance of the solution is measured from known amounts of nitrite to produce a standard curve to analyze samples. Use the data in the provided table, or from the linked spreadsheet to make a graph and perform a linear regression using treadline. mg/L NO3 Absorbance 0 0.1 0.3 0.5 0.7 o 0.183 0.542 0.914 1.298) 1.61) In order for this to work, samples must be diluted prior measurement so that the concentration falls within the range of the standard curve. If a sample underwent a 30 dilution, and produce an absorbance value of 0.953, what is the resultant concentration…arrow_forwardIn the laboratory, a student adds 14.0 g of silver nitrate to a 375 mL volumetric flask and adds water to the mark on the neck of the flask. Calculate the concentration (in mol/L) of silver nitrate, the silver ion and the nitrate ion in the solution.arrow_forwardIn the laboratory, a student dilutes 10.1 mL of a 7.85 M hydroiodic acid solution to a total volume of 125.0 mL. What is the concentration of the diluted solution? Concentration=____Marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY