
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
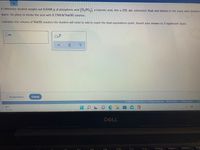
Transcribed Image Text:A chemistry student weighs out 0.0266 g of phosphoric acid (H,PO,), a triprotic acid, into a 250. mL volumetric flask and dilutes to the mark with distilled
water. He plans to titrate the acid with 0.1500M NaOH solution.
Calculate the volume of NAOH solution the student will need to add to reach the final equivalence point. Round your answer to 3 significant digits.
| mL
x10
Explanation
Check
2022 McGraw Hill LLC All Rights Reserved. Terms of Use | Privacy Center | Acc
80 F
へ
Cloudy
DELL
F11
F12
PrtScr
Insert
Delete
F8
F9
F10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A student prepares a 0.28 mM aqueous solution of acetic acid (CH,CO,H). Calculate the fraction of acetic acid that is in the dissociated form in his solution. Express your answer as a percentage. You will probably find some useful data in the ALEKS Data resource. Round your answer to 2 significant digits. |% x10arrow_forwardA chemist prepares a solution of silver perchlorate (AgClO) by measuring out 440. g of silver perchlorate into a 100. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's silver perchlorate solution. Round your answer to 3 significant digits. Ú mol/L x10 Xarrow_forwardPLEASE see IMAGE attachedarrow_forward
- A chemistry student weighs out 0.181 g of phosphoric acid (H3PO. a triprotic acid, into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.0800 M NaOH solution. Calculate the volume of NaOH solution the student will need to add to reach the final equivalence point. Round your answer to 3 significant digits. O mLarrow_forwardA chemistry student weighs out 0.0812 g of sulfurous acid (H₂SO3), a diprotic acid, into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.1500M NaOH solution. Calculate the volume of NaOH solution the student will need to add to reach the final equivalence point. Round your answer to 3 significant digits. mL x10 X S Elld ? ol Ararrow_forwardCalculating molarity using solute mass A chemist prepares a solution of sodium nitrate (NaNO3) by measuring out 95.5 g of sodium nitrate into a 250. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's sodium nitrate solution. Round your answer to 3 significant digits. mol/L x10 1/3 Ś Olivia V ? 00 Aarrow_forward
- A chemistry student weighs out 0.170 g of citric acid (H₂CH₂O₂), a triprotic acid, into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.1700 M NaOH solution. Calculate the volume of NaOH solution the student will need to add to reach the final equivalence point. Be sure your answer has the correct number of significant digits. 0mL x10 Xarrow_forwardA student prepares a 0.38 mM aqueous solution of acetic acid (CH3CO₂H). Calculate the fraction of acetic acid that is in the dissociated form in his solution. Express your answer as a percentage. You will probably find some useful data in the ALEKS Data resource. Round your answer to 2 significant digits. 11 % 0.0 x10 X S 0 Earrow_forwardA buret is used to measure the volume of FeCl3 aqueous solution. The starting volume is recorded to be 5.28 mL. The ending volume is recorded as 17.44 mL. What is the total volume of FeCl3 solution dispensed?arrow_forward
- A chemistry student weighs out 0.0623 g of acrylic acid (HCH,CHCO,) into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0:1400 M NaOH solution. Calculate the volume of NaOH solution the student will need to add to reach the equivalence point. Round your answer to 3 significant digits.arrow_forwardO STOICHIOMETRY OO Jada Dilution A chemist makes 510. mL of zinc oxalate (ZnC,0, working solution by adding distilled water to 300, mL of a 139. µM stock solution of zinc oxalate in water. Calculate the concentration of the chemist's working solution. Round your answer to 3 significant digits. Explhation Check 2021 McGraw H Education All Rights Reserved Terms of Use Privacy Accessibil esc Thank The & 1 2 4 6 8 9 tab W E R T Y { } P A os lock G H K V B N M < "ontrol option command command optionarrow_forwardAn impure sample contains 1.01 g of impurities and 4.92 g of benzoic acid. The impure sample is dissolved in water, then heated. The solution is then cooled. How many grams of benzoic acid will recrystallize when the solution has cooled? Include the unit and two decimal places in your answer. The solubilities of the impurity and benzoic acid in hot water are found as an item in the Recrystallization Experiment folder. Solubility Data for Report Questions 10 Compound Cold Water solubility Hot Water Solubility Benzoic acid 0.42 g per 100 mL of water 5.79 g per 100 mL of water Impurity 0.61 g per 100 mL of water 4.62 g per 100 mL of waterarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY