
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Just need to check answer, dont need lengthy explanation.
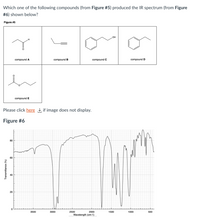
Transcribed Image Text:Which one of the following compounds (from Figure #5) produced the IR spectrum (from Figure
#6) shown below?
Figure #5
HO
compound A
compound B
compound C
compound D
compound E
Please click here , if image does not display.
Figure #6
80
60
40
20
2500
Wavelength (cm-1)
3500
3000
2000
1500
1000
500
Transmittance (%)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Heisenberg principal questions: The mass of sodium ions is 4.86 x 10-25 Kg. What will be error in velocity measurement if the error in position measurement is 1 nano-meter.(10-19 m)arrow_forwardExercise 3.35 - Enhanced - with Feedback and Hints MISSED THIS? Read Section 3.5 (Fages 100 - 105) ; Watch KCV 3.5, IWE 3.3. hydroxide Enter a formula for the compound that forms between magnesium and each polyatomic ion. Express your anewer an a chemical formula. > Vlew Avallable Hintſe) OH DA chemical reaction does not accur for this question. Submit Prevlouc Ancwers X Incorrect Try Again; 3 attempts remaining Part B chramate Exprese your answer as a chemical formula. > Vlew Avallable Hint(s) AZ ? DA chermical reaction does nat accur for this question. Submit • Part C phosphate Express your answer as a chemical formula. • Vlew Avallable Hint(e) DA chemical reaction does nat accur for this question. Submit Part D cyanide Express your answer as a chemical formula. • Vlew Avallable Hint(e) ? DA chemical reaction does not accur for this question.arrow_forwardLabQuest 17 DATA TABLE Trial 1 2 3 4 5 6 Concentration (mol/L) 0.080 0.16 0.24 0.32 0.40 Unknown number Absorbance (no units) 0.2711 0.437 1.480 1.932 2.171 0.3 DATA ANALYSIS 1. Describe an alternate method for determining the molar concentration of your unknown sample of copper (II) sulfate solution, using the standard data.arrow_forward
- CHE102 B urse Home KCHE102 Trad Exam 1 Item 6 Part A What is the mass of 3.00 L of an intravenous glucose solution with a density of 1.25 g/ml? Submit Request Answer Provide Feedback Marrow_forwardWhich of the following is true of electrostatic forces but not gravitational forces? (Select all that apply.) Are mediated by fields Require the presence of two or more objects Can be attractive or repulsive Increase as the mass of the objects increase Increase as the charge of the objects increase Increase as the distance between the objects increases Save Answerarrow_forwardCheck all that applyarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY