
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
For the following question please solve #1 letters c-i, solve and determine if its SN1, SN2, E1 or E2.
USE IMAGE AS REFERNCE
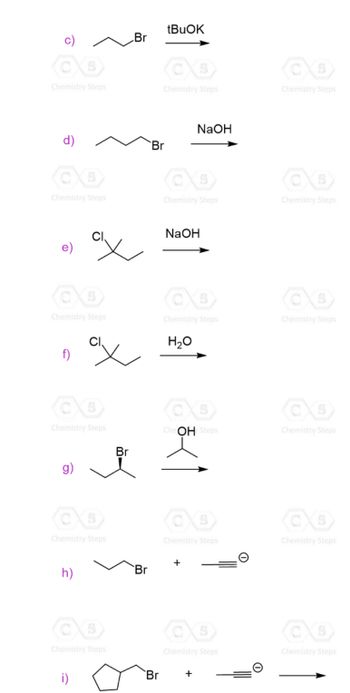
Transcribed Image Text:Chemistry Steps
d)
Chemistry Steps
Chemistry Steps
f)
g)
Chemistry Steps
ax
h)
Chemistry Steps
ax
i)
Chemistry Steps
Br
Br
Br
Br
Br
tBuOK
Chemistry Steps
Chemistry Steps
NaOH
NaOH
Chemistry Steps
H₂O
CheOH Steps
Chemistry Steps
Chemistry Steps
+
Chemistry Steps
Chemistry Steps
Chemistry Steps
Chemistry Steps
Chemistry Steps
Chemistry Steps
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can you help me on questions 2 AND 3arrow_forwardPart A Mark with the green check to indicate that the given direction of electron flow in the following set of molecules using curved arrows notation is correct and with the red X label to indicate when incorrect. Drag the appropriate labels to their respective targets. ► View Available Hint(s) X X X H₂C X CH₂ H₂C Reset Help You labeled 2 of 7 targets incorrectly. Electrons can only be shown to migrate if there is an atomic p orbital to move them to. Moving a lone pair electrons to form a new π bond can only occur if the atom acceptingarrow_forwardanswer me onlyarrow_forward
- Learning Target 3 Criteria for satisfactory score All molecular drawings must be valid Lewis structures (octet rule, formal charge, valence, geometry). Curved arrows will be interpreted very strictly. Thus, they must clearly indicate the origin and destination of electron delocalization. Tasks 1. Generate at least four reasonable resonance structures for the molecule shown in Figure 1 by drawing curved ar- rows and the resulting structure next to it (indicate reading direction by numbering structures and writing the or- der for the reader to follow). 2. Select the most significant and least significant resonance contributor and explain your choice. NH Figure 1: Compound 3arrow_forwardPlease answer all and get like Hand written are strictly prohibited.arrow_forwardCan you please help me with this?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY