
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
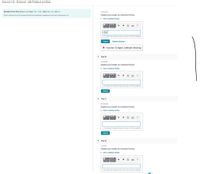
Transcribed Image Text:### Exercise 3.35 - Enhanced - with Feedback and Hints
---
#### MISSED THIS? Read Section 3.5 (Pages 100 - 108); Watch KCV 3.6, IWE 3.3.
**Task:** Enter a formula for the compound that forms between magnesium and each polyatomic ion.
---
#### Part A: Hydroxide
**Prompt:** Express your answer as a chemical formula.
**Input Area:** A chemical equation editor is provided for entering the answer.
**Answer Entered:** OH^-
**Feedback:**
- Status: Incorrect
- Message: "Incorrect. Try Again; 3 attempts remaining"
**Options:**
- View Available Hint(s)
- Submit
- Previous Answers Checkbox (A chemical reaction does not occur for this question.)
---
#### Part B: Chromate
**Prompt:** Express your answer as a chemical formula.
**Input Area:** A chemical equation editor is provided for entering the answer.
**Options:**
- View Available Hint(s)
- Submit
- Previous Answers Checkbox (A chemical reaction does not occur for this question.)
---
#### Part C: Phosphate
**Prompt:** Express your answer as a chemical formula.
**Input Area:** A chemical equation editor is provided for entering the answer.
**Options:**
- View Available Hint(s)
- Submit
- Previous Answers Checkbox (A chemical reaction does not occur for this question.)
---
#### Part D: Cyanide
**Prompt:** Express your answer as a chemical formula.
**Input Area:** A chemical equation editor is provided for entering the answer.
**Options:**
- View Available Hint(s)
- Submit
- Previous Answers Checkbox (A chemical reaction does not occur for this question.)
---
**Note:** Each part provides a chemical equation editor for the expression of answers and options to view hints or submit responses. Immediate feedback on correctness of the answer is provided upon submission.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 18 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the name for GaPO4?arrow_forwardDetermine the chemical formula for each of the compounds. sodium sulfite: N22SO3 potassium phosphate: KH2PO4 Incorrect Incorrect silver sulfate: Ag2SO4 ammonium nitrate: NH4NO3 Incorrect Incorrect Question Source: MRG - General Chemistry| Publisher: University Sc MAR 29 MacBook Air 80 DII DD F3 F7 F8 F9 F10 F11 23 24 % & 4 9. { E Y U %1 F G J K M command option .... * 00 Tarrow_forwardIdentify all of the ions/polyatomic ions in the paragraph above. Use it along with what you learned in live lesson to make your own chart.arrow_forward
- MISSED THIS? Read Section 3.9 (Pages 113 - 118) ; Watch IWE 3.16. Part A How many fluorine atoms are present in 6.05 g of C2F4? Express your answer to three significant figures. • View Available Hint(s) ? atoms Submitarrow_forwardPlease help!arrow_forwardWhat is the chemical formula for Copper (I) Sulfide? Show your steps.arrow_forward
- new-source.https://.... * Question Completion Status: The correct name for this diatomic molecule Cl₂, is O chlorine atom O dichloride dichlorine Ochlorine molecule THERENI esc QUESTION 15 Match molecules and ionic compounds with their names *FeCl2 ✓ H₂S ✓ Cu₂0 ✓ CuO ✓ NH3 NHACH ✓ H₂SO4 F Click Save and Submit to save and submit. Click Save All Answers to save all answers → # с LA A. sulfuric acid B. Iron (II) Chloride C. ammonium hydroxide D. ammonia % E. copper (II) oxide F. Hydrosulfuric acid G. copper (1) oxide M hp &arrow_forwardkeAssignment/takeCovalentActivity.do?locator-assignment-take MI 2req 2req (X) 2req 2req 2req 2req 2req 2req A compound is found to contain 7.962 % silicon, 20.10 % chlorine, and 71.94 % iodine by mass. What is the empirical formula for this compound? To answer the question, enter the elements in the order presented above. Submit Answer Use the References to access important values if needed for this question. K Retry Entire Group 9 more group attempts remaining Previous Next 14 * - 0arrow_forwardS Profiles Tab Window Help Homework X World view X Paraphrasi X signment/takeCovalentActivity.do?locator=assignment-take G Grammarly X moles How many moles of Be are there in 72.8 grams of Be ? Submit Answer My Home [Review Topics] [References] Use the References to access important values if needed for this question. X Retry Entire Group 9 more group attempts remaining OWLv2 | C X G E Show Allarrow_forward
- 15 of 16 I Review | Constants | Periodic Table Part A H O ||| CH3-C-N-CH2-CH3 Spell out the full name of the compound. Submit Request Answer Part B CH3-CH2-CH2 C-NH2 Spell out the full name of the compound. Pearson carson Education Inc. All rights reserved.| Terms of Use | Privacy Policy | Permissions | Contact Us | 12:14 AM xpジC 10/29/2020 PrtSc Delote F10 08.arrow_forwardent/takeCovalentActivity.do?locator-assignment-take Visited 9 eq req Use the References to access important values if needed for this question. 1. How many MOLECULES of nitrogen trifluoride are present in 5.36 grams of this compound? molecules. 2. How many GRAMS of nitrogen trifluoride are present in 7.20x1022 molecules of this compound ? grams. Submit Answer Retry Entire Group 9 more group attempts remaining Previous 07:10arrow_forwardPredict the chemical formula for a compound composed of Na+ and S2- and give the appropriate name.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY