
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
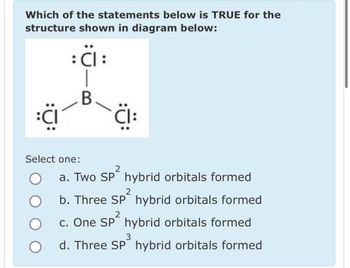
Transcribed Image Text:Which of the statements below is TRUE for the
structure shown in diagram below:
: Cl :
B
:CI
Select one:
O
CI:
2
a. Two SP² hybrid orbitals formed
2
b. Three SP² hybrid orbitals formed
2
c. One SP² hybrid orbitals formed
3
d. Three SP hybrid orbitals formed
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 7.59 What type of hybrid orbital is generated by combining the valence s orbital and all three valence p orbitals of an atom? How many hybrid orbitals result?arrow_forwardDo lone pairs about a central atom affect the hybridization of the central atom? If so, how?arrow_forwardLets look more closely at the process of hybridization. (a) What is the relationship between the number of hybrid orbitals produced and the number of atomic orbitals used to create them? (b) Do hybrid atomic orbitals form between different p orbitals without involving 5 orbitals? (c) What is the relationship between the energy of hybrid atomic orbitals and the atomic orbitals from which they are formed?arrow_forward
- Aspartame is a compound that is 200 times sweeter than sugar and is used extensively (under the trade name NutraSweet) in diet soft drinks. The skeleton structure of the atoms in aspartame is (a) Complete the Lewis structure and give the number of and bonds in aspartame. (b) What is the hybridization about each carbon atom that forms a double bond with an oxygen atom? (c) What is the hybridization about each nitrogen atom?arrow_forwardConsider acetyl salicylic acid, better known as aspirin. Its structure is (a) How many sigma and pi bonds are there in aspirin? (b) What are the approximate values of the angles marked (in blue) A, B, and C? (c) What is the hybridization of each atom marked (in red) 1, 2, and 3?arrow_forwardWhat hybrid orbitals would be expected for the central atom in each of the following molecules or ions?arrow_forward
- • explain how hybridization reconciles observed molecular shapes with the orbital overlap model.arrow_forwardWhy is the concept of hybridization required in valence bond theory?arrow_forwardMethylcyanoacrylate is the active ingredient in super glues. Its Lewis structure is (a) How many sigma bonds are in the molecule? (b) How many pi bonds are in the molecule? (c) What is the hybridization of the carbon atom bonded to nitrogen? (d) What is the hybridization of the carbon atom bonded to oxygen? (e) What is the hybridization of the double-bonded oxygen?arrow_forward
- Identify the hybrid orbitals used by antimony in SbCl5 and in SbCl6, the ion formed from the reaction of SbCl5 and Cl. Explain your choices.arrow_forwardWhen two amino acids react with each other, they form a linkage called an amide group, or a peptide link. (If more linkage. are added, a protein or polypeptide is formed.) (a) What are the hybridizations of the C and N atoms in the peptide linkage? (b) Is the structure illustrated the only resonance structure possible for the peptide linkage? If another resonance structure is possible. compare it with the o ne shown. Decide which is the more important structure. (c) The computer-generated structure shown here, which contains a peptide linkage, shows that this linkage is flat. This is an important feature of proteins. Speculate on reasons that the CONH linkage is planar. What are the sites of positive and negative charge in this dipeptide?arrow_forwardStrike-anywhere matches contain a layer of KClO3 and a layer of P4S3. The heat produced by the friction of striking the match causes these two compounds to react vigorously, which sets fire to the wooden stem of the match. KCIO3 contains the ClO3 ion. P4S3 is an unusual molecule with the skeletal structure. (a) Write Lewis structures for P4S3 and the ClO3 ion. (b) Describe the geometry about the P atoms, the S atom, and the Cl atom in these species. (c) Assign a hybridization to the P atoms, the S atom, and the Cl atom in these species. (d) Determine the oxidation states and formal charge of the atoms in P4S3 and the ClO3 ion.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
