
Introductory Chemistry For Today
8th Edition
ISBN: 9781285644561
Author: Seager
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
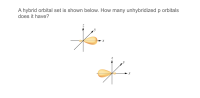
Transcribed Image Text:A hybrid orbital set is shown below. How many unhybridized p orbitals
does it have?
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give the shape that describes each hybrid orbital set: (a) sp2 (b) sp3d (c) sp (d) sp3d2arrow_forward7.59 What type of hybrid orbital is generated by combining the valence s orbital and all three valence p orbitals of an atom? How many hybrid orbitals result?arrow_forwardDetermine the bond order of each member of the following groups, and determine which member of each group is predicted by the molecular orbital model to have the strongest bond. (a) H2,H2+,H2 (b) O2,O22+,O22 (c) Li2,Be2+,Be2 (d) F2,F2+,F2 (e) N2,N2+,N2arrow_forward
- Using cartoon representations, draw a molecular orbital mixing diagram for a CO bond. In your picture, consider the relative energies of C and O and how this changes the resulting bonding and antibonding molecular orbitals relative to a CC bond.arrow_forwardLets look more closely at the process of hybridization. (a) What is the relationship between the number of hybrid orbitals produced and the number of atomic orbitals used to create them? (b) Do hybrid atomic orbitals form between different p orbitals without involving 5 orbitals? (c) What is the relationship between the energy of hybrid atomic orbitals and the atomic orbitals from which they are formed?arrow_forwardIn Chapter 6, we study a group of organic cations called carbocations. Following is the structure of one such carbocation, the tert-butyl cation. (a) How many electrons are in the valence shell of the carbon bearing the positive charge? (b) Using VSEPR, predict the bond angles about this carbon. (c) Given the bond angle you predicted in (b), what hybridization do you predict for this carbon?arrow_forward
- In sp²-hybridized orbitals, how many p-orbitals remain to form multiple bonds?arrow_forwardA o bond arises from the straight-on overlap of two atomic Sigma Bonding orbitals. The electron density lies along the axis of the two 'bonded nuclei. sp3 hybrid orbital Example: Sigma Bonding in methane, CH4 1s orbital What atomic or hybrid orbitals make up the sigma bond between Xe and F in xenon tetrafluoride, XeF4 ? orbital on Xe+ orbital on F What are the approximate F-Xe-F bond angles ? (list all possible)arrow_forwardA π bond arises from "sideways" overlap of two parallel p orbitals. The electron density lies above and below a plane containing the 2 nuclei that is perpendicular to the orbitals. 8 p-orbital atom 1 + 8 p-orbital atom 2 π bond What atomic or hybrid orbitals make up the bond between C₂ and O₁ in acetic acid, CH3COOH ? sp² orbital on C₂ + sp² orbital on 0₁ How many o bonds does C₂ have in CH3COOH ? 2 How many bonds does C₂ have? 1arrow_forward
- Sigma Bonding Ao bond arises from the straight-on overlap of two atomic orbitals. The electron density lies along the axis of the two bonded nuclei. sp3 hybrid orbital Example: Sigma Bonding in methane, CH4 1s orbital What atomic or hybrid orbitals make up the sigma bond between C, and O in acetaldehyde, CH3CHO ? (C, is the second carbon in the structure as written.) orbital on C, + orbital on O What is the approximate C,-C2-O bond angle ?arrow_forwardDescribe the bonding in the methanol molecule, CH30H, using valence bond theory. O A. The electron-pair geometry around both the C and O atoms in CH3OH is tetrahedral. Thus, we assign sp2 hybridization to each atom, and the C – O bond is formed by overlap of sp² orbitals on these atoms. Each C - H bond is formed by overlap of a carbon sp³ orbital with a hydrogen 1s orbital, and the 0 - H bond is formed by overlap of an oxygen sp3 orbital with the hydrogen 1s orbital. Two lone pairs on oxygen occupy the remaining sp3 orbitals on the atom. O B. The electron-pair geometry around both the C and O atoms in CH3OH is tetrahedral. Thus, we assign sp³ hybridization to each atom, and the C - O bond is formed by overlap of sp3 orbitals on these atoms. Each C - H bond is formed by overlap of a carbon sp? orbital with a hydrogen 1s orbital, and the 0 - H bond is formed by overlap of an oxygen sp? orbital with the hydrogen 1s orbital. Two lone pairs on oxygen occupy the remaining sp2 orbitals on the…arrow_forwardSigma Bonding Ao bond arises from the straight-on overlap of two atomic orbitals. The electron density lies along the axis of the two bonded nuclei. sp3 hybrid orbital Example: Sigma Bonding in methane, CH4 1s orbital What atomic or hybrid orbitals make up the sigma bond between C, and C, in propyne, CHCCH3 ? (C, is the second carbon in the structure as written.) orbital on C2 + orbital on C What is the approximate C,-C2-C, bond angle ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
