
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
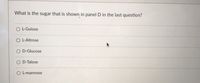
Transcribed Image Text:**Question:**
What is the sugar that is shown in panel D in the last question?
**Options:**
- ○ L-Gulose
- ○ L-Altrose
- ○ D-Glucose
- ○ D-Talose
- ○ L-mannose
(Note: There are no graphs or diagrams present in the image that require explanation.)

Transcribed Image Text:### Understanding the Structure of Gulose
#### Open Chain Form of Gulose
The image illustrates Gulose in its open chain form, which is depicted as follows:
- **CHO** at the top.
- The carbon atoms are connected vertically with alternating groups:
- **C** connected to an **H** and **OH**.
- **C** connected to an **H** and **OH**.
- **C** connected to an **HO** and **H**.
- **C** connected to an **H** and **OH**.
- At the bottom, connected to the last carbon is **CH2OH**.
#### Identifying β-D-Gulose
The question posed is: “Which of the following shows β-D-Gulose?”
There are five cyclic structures labeled from A to E:
- **A)** A six-membered ring with OH groups in varying positions.
- **B)** A six-membered ring similar to A, with different OH and H orientations.
- **C)** Another six-membered ring; features differ in the spatial arrangement of OH and H groups.
- **D)** Another structural variant with a distinct orientation of OH and H groups.
- **E)** Features a different configuration of OH and H groups from the other options.
Each structure includes:
- A ring structure symbolizing the cyclic form.
- Functional groups attached at different points indicating specific stereochemistry.
The task is to match these structures to the β-anomeric form of D-Gulose by examining the orientation of the hydroxyl groups in each cyclic structure.
Expert Solution
arrow_forward
Step 1
Carbohydrates are polyhydroxy aldehydes or ketones which are associated with reducing property and each carbohydrate is composed of carbon, hydrogen, and oxygen which are arranged in the empirical formula CnH2nOn.
Step by stepSolved in 7 steps

Knowledge Booster
Similar questions
- From the SDS for 4-nitrobenzaldehyde, what does the Hazard Code H317 refer to? 1) Skin sensitisation (Category 1) 2) Explosive; mass explosive hazard 3) Contains gas under pressure; may explode if heated O 4) May cause drowsiness or dizzinessarrow_forwardWhat process is taking place in the illustration? Supply the appropriate labels for all numbered parts of the illustration.arrow_forwardThis is a portion of the periodic table of elements. What is the atomic mass of arsenic (As)? 3 Li 6.941 11 Na 22.99 19 K 39.10 18 26.98 39.95 74.92 33 4 Be 9.012 12 Mg 24.31 5 B 10.81 13 Al 26.98 6 C 12.01 14 Si 28.09 20 31 32 Ca Ga Ge 40.08 69.72 72.59 7 N 14.01 8 O 16.00 15 16 P S 30.97 32.07 9 F 19.00 17 Cl 35.45 33 34 35 As Se Br 74.92 78.96 79.90 10 Ne 20.18 18 Ar 39.95 36 Kr 83.60arrow_forward
- This test will differentiate between aromatic amino acids with an acitivated ring from other amino acids. a) Nihydrin Test b) Xanthoproteic Test C) solubility test d) polarimeter testarrow_forwardThe cofactor shown below: is an oxidizing agent is a reducing agent is a carrier of acyl groups is flavin mononucleotide The cofactor shown below: CH2 HC OH HC OH HC OH CH2 H₂C NH H H H OH OH NH₂ 1) is an oxidizing agent 2) is a reducing agent 3) is a carrier of acyl groups 4) is flavin mononucleotidearrow_forward11.3 a). What is the unit used for measuring ozone layers? b). What is the wavelength range of the UV radiation? c). How is ozone different from oxygen? d). When CFCs are exposed to UV or sun light, what species are produced? e). What is the role of chlorine radical in the ozone formation or reactions. f). What in the polar zone makes the depletion of ozone more serious?arrow_forward
- SDS coats proteins with a: A) Positive Charge B) Negative Chargearrow_forwardClassify the monosaccharides. H H-C- -OH H-C- -OH H- CH₂OH D-erythrose H- CH₂OH FO -OH H. CH₂OH D-erythrulose H- -OH -OH H-C OH CH₂OH H- D-ribose CH₂OH OH H-C OH CH₂OH D-ribulose H H-C- CH₂OH D-glyceraldehyde -OH HO- -C- H- CH₂OH FO -H -C- -OH H-C OH CH₂OH D-fructose CH₂OH C=O CH₂OH Dihydroxyacetone triose Answer Bank ketose hexose aldose tetrose pentosearrow_forwardFor the following molecule, I need to identify several things, but I don't understand how to go about it. a) anomeric carbon b) carbon 1 c) carbon 5 d) which oxygen atoms in a hydroxyl group would point to the right in a Fischer Projectionarrow_forward
- A) what does the figure illustrate? B) Label the components in the figure pointed by the arrows and briefly mention the bonding partners involved in each casearrow_forward.1 Given structures (Betaxlol) and (Timolol), which one would be a better choice topically to treat glaucoma in a patient with a history of asthma? Provide a SBTE for your answer. cr 0- CH2-CH-CH2-NH2-CH(CH3)2 -H2C-0-H2C-H2C- ÓH (Betaxolol) -00, c=C, H H -o-CH2-CH-CH2-NH2-C(CH3)3 (Timolol) OHarrow_forwardName the nitrogenous bases that are bondedarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON