
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
A) what does the figure illustrate?
B) Label the components in the figure pointed by the arrows and briefly mention the bonding partners involved in each case
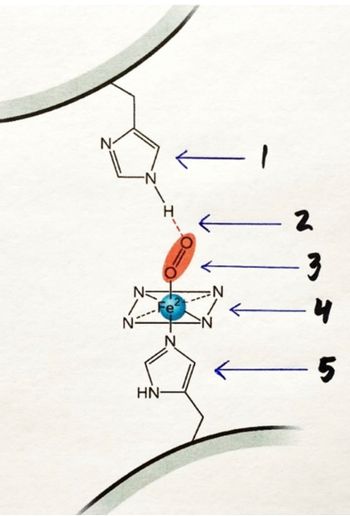
Transcribed Image Text:The image shows a molecular model with labeled components:
1. **Imidazole Ring**: The first component pointed out is an imidazole ring, represented by a five-membered aromatic ring with two nitrogen atoms.
2. **Hydrogen Bond**: This is denoted by a dashed line, indicating a hydrogen bond connecting the imidazole ring to another part of the molecule.
3. **Oxygen Molecule (O2)**: Shown as a red ellipse with two oxygens, indicating the presence of a diatomic oxygen molecule.
4. **Iron (Fe2+) Center**: Represented by a blue sphere, this indicates the iron ion (Fe2+) at the center, coordinating with nitrogen atoms.
5. **Second Imidazole Ring**: Another imidazole ring similar to the first one, suggests a symmetric structure or coordination.
This diagram is likely illustrating a heme group or similar coordination complex, where iron (Fe2+) is central, bonded to an oxygen molecule. Imidazole rings typically represent histidine residues in biological contexts.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Draw Ramachandran plot for: a) regular secondary structure with Φ = 60-65 and Ψ = 60-80 b) regular secondary structure with Φ = -170 and Ψ = 170 c) intrinsically disordered proteins d) explain why these Ramachsndran plots will be different, and what secondary structures are described in A and in Barrow_forwardDraw the following amino acids described below include all hydrogens in the structure. 1)amino acid Proline at pH2.0. 2) amino acid glycine at pH 3.0. 3) amino acid with a Methionine at pH 7.0. 4) the amino acid Histidine at pH5.0.arrow_forwardThe naturally occurring opioid met-enkephalin is a peptide with the structure Tyr- Gly-Gly-Phe-Met. What is its net charge of this molecule in the body at pH 7. A) +1 B) +2 C) O D) -1 E) -2arrow_forward
- Which one of the following types of bond is principally responsible for holding the α-helix shape of a protein secondary structure : A) peptide B) disulfide C) hydrogen D)ionicarrow_forwardDescribe, identify, and DRAW the monomers (e.g. what is typically illustrated as a hexagon?) andpolymers of:a. Proteinsb. Carbohydratesc. Lipids Identify the type of chemical bonds that join the monomers of:a. Proteins (describe the structure and function of a polymer)b. Carbohydrates (describe the structure and function of a polymer)c. Lipids Identify the portions of the cell membrane (phospholipid bi-layer) that area. Hydrophobicb. Hydrophilicc. Polard. Non-polararrow_forwardConsider the following fatty acid attached below. a) What is the number convention for this fatty acid above, including the location of the double bond from the α carbon end? Remember to use the Δ (delta) in your answer for the double bond location b) What is the ω (omega) numbering of the double bond for the fatty acid above? c) What is the product after two hypothetical additional rounds of synthesis for the fatty acid? Remember that synthesis adds 2 carbons at a time to the carboxylic end.arrow_forward
- 1. A) Draw a guanylate nucleotide and label the three main structural features B) Does this contain a purine or pyrimidine base? C) Would this nucleotide be found in DNA or RNA? Why? D) To which carbons of the furanose sugar are the other important structural features attached? E) Draw an adenylate residue bonded to the guanylate from below as it would be in a nucleic acid chain F) Label the phosphodiester bond G) Label which side of your dinucleotide is the 5' and 3' side H) Draw the nucleotide residues that base pair with guanylate and adenylate in the position and location that would permit pairing 1) Draw the hydrogen bonds that occur in both pairings.arrow_forwardA binding protein binds to a ligand L with a Kd of 400 nM. How much ligand is present when Y is (a) 0.25, (b) 0.6, (c) 0.95?arrow_forward1. What is the isoelectric point (pI) of lysine which has pKa values of 2.1 for the α carboxyl group, 9.7 for the α amino group and 10.5 for the side chain amino group? 2. Which of the following is most likely to be found on the exterior of a protein? A) Pro B) Trp C) Ser D) Glu 3. The type of reaction that forms a peptide bond is A) Elimination B) Hydrolysis C) Nucleophilic substitution D) Condensationarrow_forward
- Having peptides arranged in a beta sheet would be an example of a secondary structure A) True B) Falsearrow_forwardProper folding is essential for most proteins to function. Which of the following statement about protein folding are correct. There may be more than 1 right answer. a) changing the primary sequence will change the final conformation b) desaturation results in a protein that has a higher free energy than the native conformation c) protein spontaneously fold into their correct shape d) denatrutation will cause a protein to lose its tertiary structurearrow_forwardThis amino acid is: (Note that the non-ionized form is shown; remember that backbone groups are irrelevant to classification) a) Polarized (+) charged b) Nonpolar c) Polar and (-) charged d) Polar unchargedarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON