
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
Which of the following amino acids will bind to anion exchange resin at pH 7.0?
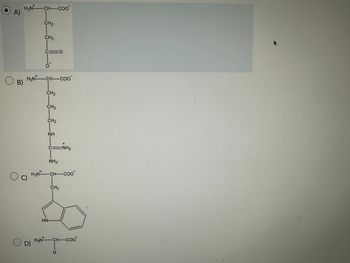
Transcribed Image Text:A)
H₂N-CH-Coo
O B)
O C
CH₂
CH₂
H3NCH-COo
O. D)
C10
CH₂
CH₂
CH₂
ΝΗ
CINH₂
NH₂
H3NCH-COo
CH₂
HN-
H₂N-CH-Coo
H
Expert Solution
arrow_forward
Step 1: Anion-exchange chromatography
Anion-exchange chromatography is a type of ion exchange chromatography. Here the stationary phase has positively charged resins called anion exchange resins. The resins are called so because they can bind to substances with a net negative charge.
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Similar questions
- What is the TOTAL NET CHARGE of a free arginine amino acid at pH = 12.5?arrow_forwardUronic acids are another class compound for carbohydrates. a) Describe the structural difference between glucose and glucouronic acid (in either the Fischer or Haworth form) b) Describe the saccharide units and glycosidic bonding found in hyaluronic acid.arrow_forward1) Find the pH of 0.1 M of the differnet forms histidine species. (See image for equation and pKa values) 2) What is the principal specis at pH 1, pH 5, pH 8, and pH 11?arrow_forward
- Draw two different possible hydrogen-bonding interactions between two molecules of formamide (HCONH2). Clearly label the hydrogen-bond donor and acceptor atoms. Which of these two possible hydrogen-bonding interactions is more likely to occur? (Hint: Consider resonance structures for formamide.)arrow_forwardWhat is the principal form of arginine at pH 8.0? Approximately what fraction is in this form?arrow_forwardWhich of the twenty naturally occurring amino acid side chains are charged at a pan of 1.00? pH of 7.00? pH of 12.00? Make a table.arrow_forward
- What is Pindolol durg stability in various ph, temperature, and other condition?arrow_forwardDraw the condensed structural formula of a gycerophospholipid trat contains two stearic acids and a phosphate banded to ethanolumine.arrow_forwardPhosphate buffers are commonly used to mimic biological systems. Given that phosophoric acid is a triprotic acid with three pKas (2.12, 7.21, and 12.32), why do you think this is? What are the dominant buffering compounds present in a phosphate buffer near physiological pH?arrow_forward
- What is the predominant ionic form of ribose-5-phosphate at physiological pH? Would ribose-5-phosphate be a good biological buffer for cells?arrow_forwardA tetrameric protein dissociates into dimers when the detergent sodium dodecyl sulfate (SDS) is added to a solution of the protein. But the dimers are termed SDS-resistant because they do not further dissociate into monomers in the presence of the detergent. What intermolecular forces might be acting at the dimer-dimer interface? Are the intermolecular forces acting at the monomer-monomer interface different? Explain.arrow_forwardWhat is the pH of a buffer solution that is 0.20 M proprionic acid (HC3H4O2) and 0.1 M sodium proprionate (NaC3H4O2)? The KA of proprionic acid is 1.3 x 10-5arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON