
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
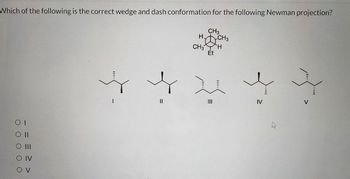
Transcribed Image Text:Which of the following is the correct wedge and dash conformation for the following Newman projection?
CH3
HCH3
CH3 H
Et
O II
O III
IV
OV
ہد ہد ہد ہد ہد
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Similar questions
- Following is a planar hexagon representation for one isomer of 1,2,4-trimethylcyclohexane. Draw the alternative chair conformations of this compound and state which of the two is more stable.arrow_forwardWhat is the correct coefficient for oxygen that would balance the following combustion reaction? O 11 O 13 O 15 O 17 O 19 2 ? 0₂ 12 CO₂ + 14 H₂Oarrow_forwardIn the lowest energy chair conformation of cis-1,3-dimethylcyclohexane, how many axial positions are occupied by hydrogen atoms? * O 2arrow_forward
- Which of the following is the correct wedge and dash conformation for the following Newman projection? 01 O 11 O III O IV OV CI -- CI || CH HD CH3 CH₂ Cl Et پاک خیار CI Varrow_forwardComplete the given chair conformation of cyclohexane by adding the listed substituents to the specified position and bond direction. Substituent Position Bond direction CH3 CH3 OH F 3 6 1 3 axial axial equatorial equatorialarrow_forwardWhich of the following is the correct Newman projection for the following compound as viewed down the indicat conformation shown? CO II O III O IV OV CH3 CH3 CH31 CH3 CI || Br Br H CH3 CH3 ||| Br CH3 CH3 IV CI Br CH3arrow_forward
- Draw a Newman projection for two more staggered conformations of this molecule. Which of your conformations is most stable? Assume that -OH and -CH3 are comparable in size.arrow_forwardWhich of the following is the correct wedge and dash conformation for the following Newman projection? Et CH3 CH3 HEt H ΟΙ ○ II III IV Ov || III =arrow_forwarddraw a stable conformation of this compoundarrow_forward
- In the most stable chair conformation of the cyclohexane below, how many methyl groups would be axial? H3C O one all three zero two CH3 CH3arrow_forwardSighting down the Ci-C2 bond axis of 1-bromopentane, Br-CH2-CH2CH2CH»CH3, draw the Newman projection of a) the anti staggered conformation b) the gauche staggered conformation c) the two eclipsed conformations. Which eclipsed structure would you expect to have the highest strain energy?arrow_forwardIn the most stable chair conformation of the cyclohexane below, how many methyl groups would be axial? CH3 CH3 three zero two all four CH3 CH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning