
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
! ( please give detail explanation)
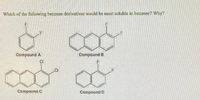
Transcribed Image Text:Which of the following benzene derivatives would be most soluble in benzene? Why?
Compound A
Compound B
CI
Compound C
Compound D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- (a) dispersion force Dispersion forces are (Very weak),(Very strong) electrostatic attractions that occur due to the random electronic motion within all substances,(excluding),(including) those that are nonpolar. When the electrons within a molecule or atom are distributed asymmetrically about the nucleus, that molecule or atom will adopt a (temporary),(permanent), (induced),(instantaneous) dipole. The presence of this dipole can then distort the electrons of a neighboring atom or molecule, producing an (induced),(instantaneous) dipole. These two rapidly fluctuating dipoles thus result in a brief electrostatic (attraction),(Repulsion) between the two species. These forces are stronger in (Larger and heavier),(Smaller and lighter) atoms and molecules. For example, dispersion forces between (Fluorine),(iodine) molecules will be stronger than dispersion forces between (Fluorine),(iodine) molecules. (b) dipole-dipole attraction A dipole-dipole force is the electrostatic attraction…arrow_forwardOnly typed solution. What is the namearrow_forwardPeardeck Exarcase use #5, Given whats below find all [],'s and calculote K [], O.100 Charge 0,010 Students, draw anywhere on this slide! Pear Deck Interactive Slide Do not remove this bar Slide 1/1arrow_forward
- This layout is confusing, can you rewrite this because this is not helpful.arrow_forwardIf enough of a monoprotic acid is dissolved in water to produce a 0.0111 M solution with a pH of 6.40, what is the equilibrium constant, Ka, for the acid? K₁arrow_forwardIn this activity, you will begin to use IR spectroscopy to examine functional groups in organic molecules. First, thinking about what an IR spectrum looks like... An IR spectrum is a most often a plot of... ✓ Choose... wavenumver vs absorbance absorbance vs wavelength transmittance vs wavenumber signal amplitude vs ppmarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY