
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
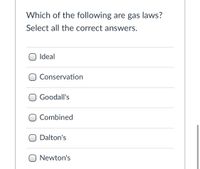
Transcribed Image Text:Which of the following are gas laws?
Select all the correct answers.
Ideal
O Conservation
O Goodall's
O Combined
Dalton's
O Newton's
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- n electronic vacuum tube was sealed off during manufacture at a pressure of 1.8 x 10-5 mm of Hg and at 27°C. Its volume is 250 mL. How many gas molecules remain in the tube? 2.45 x 1013 molecules 2.40 x 1010 molecules 1.45 x 1014 molecules 14.50 x 1016 moleculesarrow_forwardWhich of these properties is/are characteristic(s) of gases? O High compressibility. O Relatively large distances between molecules. O Formation of homogeneous mixtures regardless of the nature of gases O All of the choices.arrow_forward27. Mass Volume Circle all of the following that are the SAME for the two samples of gas represented above: Number of gas particles Total number of atoms Temperature Pressure Carbon Monoxide (CO) 8 moles 25 °C 5.0 L Density Molecular Speed (avg) Kinetic Energy (avg) Danger Argon (Ar) 8 moles 25 °C 5.0 L (Please type answer no write by hend)arrow_forward
- A Mylar™ party balloon is inflated with 4.62 L of helium in an indoor environment where the temperature is 25 °C. It is then taken outside for a winter carnival where the temperature is -15 °C. What is the volume of the balloon when it is outside? (Assume constant P.)arrow_forwardIn this experiment, we will be using the gas inside of a small hand-held lighter. Before the trial, the lighter weighed 46.747 g. After the trial the mass of the lebter was 31.104 g. what is the mass in grams of the gas used? Enter a numeric answer only. A Moving to another question will save this response. «< Question 6 of 10 8:39 AM search 耳 a 22 4/13/2022 hp insert prt sc delete 144 %24 4 %23 & L. 8 6. %3D backspace hor R T Y U P F G L enter D pause t sh C alt ctriarrow_forward2. During the experiment, you collect the following data: Mass of flask: 80.001 Mass of flask + unknown condensed liquid: 80.452 Temperature of water bath: 100 °C Pressure of gas in our system: 0.998 atm Volume of gas in our system: 265 mL How many moles of gas were produced? Hint: Do not forget to convert units as needed. Group of answer choices: A) 0.00864 moles B) 32.2 moles C) 0.0322 moles D) 8.64 molesarrow_forward
- According to Charles' Law, the temperature and volume of an ideal gas are inversely proportional. True O Falsearrow_forwardConvert 84.00 Fahrenheit to Kelvin.arrow_forwardAccording to Avogadro's law, how will the number of molecules in 2 liters of hydrogen gas compare with the number of molecules in 2 liters of oxygen gas at the same temperature and pressure? Answer in a complete sentence. H + Normal Enter your answer here **** √x = 201 BIUS X₂ X² EEAAarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY