
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Which of the ff is a glycoside?
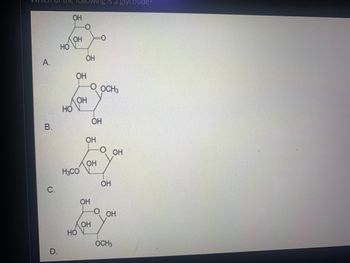
Transcribed Image Text:VVTHICHT
А.
B.
с.
D.
НО
ОН
ОН
НО
ОН
ОН
ОН
H3CO
НО
ОН
ОН
ing is a glycoside?
ОН
ОН
ОН
ОН
ОН
ОН
OCH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help answer the question on natural polymers polysaccharides 1) what is the predominant functions group in polysaccharides 2) what types of covalent linkage connects the monomers 3) compare and contrast the structure and function of startch and glycogen proteins 1) sketch the two amino acids. Contrast the polarity of the side chains Dna 1) what are the predominant groups in dna 2) the covalent linkage between nucleotides is called a phosphodiester bond. Sketch the linkagearrow_forwardthe formula of most common monosaccharide glucose is C6H12O6 then why do the resulting disaccharide have the formula C12H22O11 rather than C12H24O12arrow_forwardis this monosaccharide aldose or ketose? what is the chemical group and its location that allows its classification.arrow_forward
- Which monosaccharides form: a) lactose b) maltose c) sucrosearrow_forwardif the disaccharide maltose is formed from two glucose monosaccharides which are hexose sugars how many atoms of carbon hydrogen and oygen does maltose contain and why?arrow_forward`Of the choices listed below, which would be classified as a polysaccharide? glucose sucrose cellulose glycogen both cellulose and glycogenarrow_forward
- How to distinguish between constitutional isomer, diasteromer, enantilmer, or identical molecules?arrow_forwardBetween CH3CH2NH2 , CH3CH3 , and CH3CH2Br, which would be least soluble in water?Explain.arrow_forwardLactose is a disaccharide created by the bonding of which two monosaccharidesarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY