
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please help answer the question on
polysaccharides
1) what is the predominant
2) what types of covalent linkage connects the monomers
3) compare and contrast the structure and function of startch and glycogen
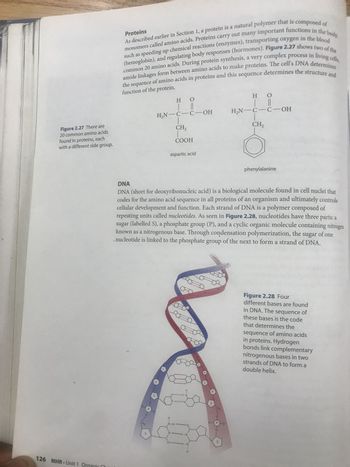
proteins
1) sketch the two amino acids. Contrast the polarity of the side chains
Dna
1) what are the predominant groups in dna
2) the covalent linkage between
Transcribed Image Text:Activity 2.2
Polystyrene is found in many products because it is
lightweight, a good insulator, and inexpensive. It is used to
make insulation for houses and cups that keep beverages
hot or cold as well as in shipping materials. However, the
manufacture of polystyrene requires hazardous chemicals
and also uses petroleum, a non-renewable resource.
Environmentally friendly Alte
Materials
• reference books
computers with Internet access
Procedure
1. Research polystyrene in order to better understand
what it is and how it is made. Use the questions below to
guide your research.
What is the chemical structure of polystyrene and
what is the chemical reaction for the production of
polystyrene?
• What properties of polystyrene make it hazardous to
the environment?
.
What is the environmental impact of polystyrene on
the environment through its manufacture, use, and
disposal?
Learning Check
19. Identify and describe the chemical process used to
convert petroleum into petrochemicals.
20. Why do manufacturers want to convert the
components in petroleum into petrochemicals?
21. Use a graphic organizer to show the steps involved
in converting ethene into PVC.
• What is the impact of polystyrene on human health?
2. Find out how is polystyrene recycled. Are there
industries in your community or in Ontario that recycle
3. Research alternatives that are available that could be
used instead of polystyrene.
Questions
1. Summarize the advantages and disadvantages of the use
of polystyrene.
2. Construct a table to show the advantages and
disadvantages associated with recycling polystyrene.
124 MHR-Unit 1 Organic Chemistry
3. Identify the advantages and disadvantages of the
alternatives to polystyrenes.
4. Hold a debate in your class about whether or not
the use of polystyrene should be banned in your
community. Invite other classes to view the debate and
judge the winners.
22. List the advantages and disadvantages to producing
PVC.
23. What are dioxins?
24. Identify two ways you can reduce the amount of
plastic waste generated in your home.
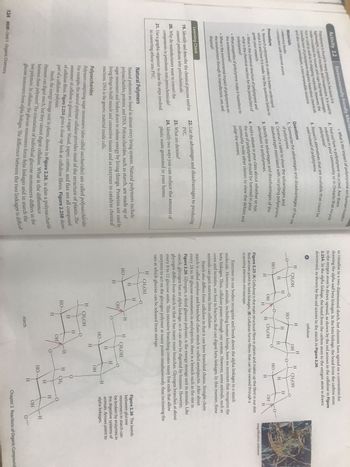
Natural Polymers
Natural polymers are found in almost every living system. Natural polymers include
polysaccharides, proteins, and DNA. Polysaccharides, such as starch, are made up of
sugar monomers and broken down to release energy by living things. Proteins are used by
living things to build muscle and connective tissues and as enzymes to catalyze chemical
reactions. DNA is the genetic material inside cells.
Polysaccharides
Polymers comprising sugar monomers (also called saccharides) are called polysaccharides.
For example, the natural polymer cellulose provides most of the structure of plants. The
monomer of cellulose is glucose, a sugar. Wood, paper, cotton, and flax are all
composed
of cellulose fibres. Figure 2.25A gives a close-up look at cellulose fibres. Figure 2.25B shows
part of a cellulose polymer.
Starch, the energy storage unit in plants, shown in Figure 2.26, is also a
Humans can digest starch, but they cannot digest cellulose. What is the difference
polysaccharide.
between these polymers? The orientation of individual glucose monomers differs in the
two polymers. In cellulose the glucose monomers form beta linkages and in starch the
glucose monomers form alpha linkages. The difference between the two linkages is difficult
to visualize in a two-dimensional sketch, but chemists have agreed on a convention for
drawing the alpha and beta linkages. In the beta linkage, the bond from the carbon atom
to the oxygen atom is drawn upward, as shown by the red arrows in the cellulose in Figure
2.25A. In the alpha linkage, the bond from the carbon atom to the oxygen atom is drawn
downward, as shown by the red arrows in the starch in Figure 2.26.
cellulose
A
H
HO
CH₂OH
H
H
HO
H
H
OH
H
H
H
HO-
OH
H
Figure 2.25 (A) Cellulose is the main structural fibre in plants and makes up the fibre in our diet.
Red arrows point to beta linkages. (B) Cellulose forms fibres that can be viewed through a
scanning electron microscope.
OH/
H
H
HO
-H
H
0
Enzymes in our bodies recognize and break down the alpha linkages in a starch
molecule. However, animals, including human beings, have no enzymes that recognize the
beta linkages. Thus, cellulose passes through our system. However, some animals, such as
cows and termites, are host to bacteria that can digest beta linkages. In this manner, these
animals can gain nutrients from cellulose.
H
Starch also differs from cellulose in that it can have branched chains. Straight-chain
starch is called amylose and branched-chain starch is called amylopectin. After about
every 24 to 30 glucose monomers in amylopectin, there is a branch such as the one in
Figure 2.26. Glycogen, a third glucose polymer, is the energy storage unit in animals. Like
starch, glycogen has an alpha linkage, so it can also be digested by humans. However,
glycogen differs from starch in that it has many more branches. Glycogen branches at about
every 8 to 12 glucose units. This extensive branching creates many free ends that allow
enzymes to act on the glycogen polymer at many points simultaneously, thus increasing the
rate at which glucose can be released from storage.
H
CH₂OH
H
CH₂OH
CH₂OH
H
starch
H
H
OH
H
HO-
H
CH₂OH
OH/
H
H
HO-
H
-H
H
CH₂OH
OH
H
H
H
HO-
O
H
H
CH₂
H
H
H
Figure 2.26 The bonds
between glucose
monomers in starch can
be broken by enzymes in
the digestive systems of
animals. Arrows point to
alpha linkages.
OH
H
B
0-
magnification: unknown
Chapter 2 Reactions of Organic Compound
Transcribed Image Text:Activity 2.2
Polystyrene is found in many products because it is
lightweight, a good insulator, and inexpensive. It is used to
make insulation for houses and cups that keep beverages
hot or cold as well as in shipping materials. However, the
manufacture of polystyrene requires hazardous chemicals
and also uses petroleum, a non-renewable resource.
Environmentally friendly Alte
Materials
• reference books
computers with Internet access
Procedure
1. Research polystyrene in order to better understand
what it is and how it is made. Use the questions below to
guide your research.
What is the chemical structure of polystyrene and
what is the chemical reaction for the production of
polystyrene?
• What properties of polystyrene make it hazardous to
the environment?
.
What is the environmental impact of polystyrene on
the environment through its manufacture, use, and
disposal?
Learning Check
19. Identify and describe the chemical process used to
convert petroleum into petrochemicals.
20. Why do manufacturers want to convert the
components in petroleum into petrochemicals?
21. Use a graphic organizer to show the steps involved
in converting ethene into PVC.
• What is the impact of polystyrene on human health?
2. Find out how is polystyrene recycled. Are there
industries in your community or in Ontario that recycle
3. Research alternatives that are available that could be
used instead of polystyrene.
Questions
1. Summarize the advantages and disadvantages of the use
of polystyrene.
2. Construct a table to show the advantages and
disadvantages associated with recycling polystyrene.
124 MHR-Unit 1 Organic Chemistry
3. Identify the advantages and disadvantages of the
alternatives to polystyrenes.
4. Hold a debate in your class about whether or not
the use of polystyrene should be banned in your
community. Invite other classes to view the debate and
judge the winners.
22. List the advantages and disadvantages to producing
PVC.
23. What are dioxins?
24. Identify two ways you can reduce the amount of
plastic waste generated in your home.
Natural Polymers
Natural polymers are found in almost every living system. Natural polymers include
polysaccharides, proteins, and DNA. Polysaccharides, such as starch, are made up of
sugar monomers and broken down to release energy by living things. Proteins are used by
living things to build muscle and connective tissues and as enzymes to catalyze chemical
reactions. DNA is the genetic material inside cells.
Polysaccharides
Polymers comprising sugar monomers (also called saccharides) are called polysaccharides.
For example, the natural polymer cellulose provides most of the structure of plants. The
monomer of cellulose is glucose, a sugar. Wood, paper, cotton, and flax are all
composed
of cellulose fibres. Figure 2.25A gives a close-up look at cellulose fibres. Figure 2.25B shows
part of a cellulose polymer.
Starch, the energy storage unit in plants, shown in Figure 2.26, is also a
Humans can digest starch, but they cannot digest cellulose. What is the difference
polysaccharide.
between these polymers? The orientation of individual glucose monomers differs in the
two polymers. In cellulose the glucose monomers form beta linkages and in starch the
glucose monomers form alpha linkages. The difference between the two linkages is difficult
to visualize in a two-dimensional sketch, but chemists have agreed on a convention for
drawing the alpha and beta linkages. In the beta linkage, the bond from the carbon atom
to the oxygen atom is drawn upward, as shown by the red arrows in the cellulose in Figure
2.25A. In the alpha linkage, the bond from the carbon atom to the oxygen atom is drawn
downward, as shown by the red arrows in the starch in Figure 2.26.
cellulose
A
H
HO
CH₂OH
H
H
HO
H
H
OH
H
H
H
HO-
OH
H
Figure 2.25 (A) Cellulose is the main structural fibre in plants and makes up the fibre in our diet.
Red arrows point to beta linkages. (B) Cellulose forms fibres that can be viewed through a
scanning electron microscope.
OH/
H
H
HO
-H
H
0
Enzymes in our bodies recognize and break down the alpha linkages in a starch
molecule. However, animals, including human beings, have no enzymes that recognize the
beta linkages. Thus, cellulose passes through our system. However, some animals, such as
cows and termites, are host to bacteria that can digest beta linkages. In this manner, these
animals can gain nutrients from cellulose.
H
Starch also differs from cellulose in that it can have branched chains. Straight-chain
starch is called amylose and branched-chain starch is called amylopectin. After about
every 24 to 30 glucose monomers in amylopectin, there is a branch such as the one in
Figure 2.26. Glycogen, a third glucose polymer, is the energy storage unit in animals. Like
starch, glycogen has an alpha linkage, so it can also be digested by humans. However,
glycogen differs from starch in that it has many more branches. Glycogen branches at about
every 8 to 12 glucose units. This extensive branching creates many free ends that allow
enzymes to act on the glycogen polymer at many points simultaneously, thus increasing the
rate at which glucose can be released from storage.
H
CH₂OH
H
CH₂OH
CH₂OH
H
starch
H
H
OH
H
HO-
H
CH₂OH
OH/
H
H
HO-
H
-H
H
CH₂OH
OH
H
H
H
HO-
O
H
H
CH₂
H
H
H
Figure 2.26 The bonds
between glucose
monomers in starch can
be broken by enzymes in
the digestive systems of
animals. Arrows point to
alpha linkages.
OH
H
B
0-
magnification: unknown
Chapter 2 Reactions of Organic Compound
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following statement about the conformation of protein is true? a. Denaturation of protein causes the changes in the primary structure of the protein b. Primary structure of protein refers to the sequence of amino acid linked by hydrogen bonds c. Alpha-helix and beta-sheet are two types of secondary structures found in proteins d. Amino acids are covalently linked by glycosidic bondsarrow_forwardSubstances originating in plant or animal material and soluble in non-polar organic solvents, due to long hydrocarbon chains, are classified as A) całbohydrates. B) lipids. C) nucleic acids. D) proteins. E) amino acids. 8&arrow_forwardWhat principles define large polysaccharides, proteins, and nucleic acids?arrow_forward
- Why do structural polysaccharides link their monosaccharide chains side by side? To store excess energy 0.00 To maintain the morphology of an organism To provide rigidity to the cell To protect the cell from water lossarrow_forwardWhy would we identify some compounds like this as a carbohydrate?arrow_forwardGlucose has which functional group?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY