
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
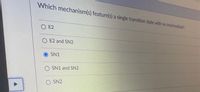
Transcribed Image Text:Which mechanism(s) feature(s) a single transition state with no Intermediate?
O E2
O E2 and SN2
SN1
SN1 and SN2
SN2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- it is known that a drug has a first order elimination rate constant of 0.17 h-1. If this drug is administered via a constant rate IV infusion, how long does it take for the plasma drug concentration to reach a steady state?arrow_forwardThe following alcohols react with HBr to afford an alkyl bromide. Based on their structures, indicate if the reaction mechanism is likely to be Sn1 or Sn2arrow_forwardWhat will the intermediate form of this molecule be after an intramolecular Sn2 attack? \N- (a) CI -NⓇ CI CI CI -CI (b) CI CI 27 (c) متر CI (d) CI Narrow_forward
- Which is the correct depiction of the transition state for the reaction shown below? a с A b B Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. с H3CO H3CO. d D CH3 e C&D CH3 possible transition states CH3 CH3 CH3 CH3 H3CO H3CO H3CO H3CO yox yox sxx sx H3CO H3CO H3CO CH3 CH3 CH3 CH3 C D A B A H3CO H3CO, CH3 CH3arrow_forwardChoose one.arrow_forwardThe two-step mechanism for the formation of products is shown below: Y M + E (slow) X + M H + F (fast) What is the intermediate in the reaction? O M O Y OFarrow_forward
- Answer the following questions associated with the mechanism below:Step 1: 2NO2 NO3 + NO (slow)Step 2: NO3 + CO NO2 + CO2 (fast)a. What is the overall reaction associated with this mechanism?b. Identify any/all intermediates in the proposed mechanism:c. What is the molecularity of the 1st step of the rxn? d. What is the molecularity of the 2nd step of the rxn? e. Which step of the rxn is the rate limiting (determining) step? f. Write an expression for the rate law associated with this mechanism in the space providedarrow_forwardWhich of the following represents the transition state of the rate- determining step in the substitution reaction between tert-butyl bromide and methanol? H3C CH, H,C CH, Br sOc--------Br 1 H3C 2 H3C CH: CH3 H3C CH, Br -Br а. 1 b. 2 с. 3 d. 4 a O barrow_forward1. Which one of the following four schemes (A-D) represents a step in the mechanism of the reaction in the box? Cl2, light (hv) + HCI CỈ H Cl-H A B H C D H B OD OC O Aarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY