
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Choose one.
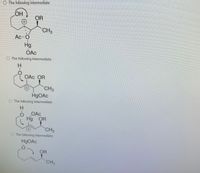
Transcribed Image Text:The image contains a series of chemical structures, each labeled as "The following intermediate." These intermediates appear to be part of a reaction sequence involving mercury acetate (Hg(OAc)₂) as a reagent. Here's a description of each:
1. **First Structure:**
- Features a cyclic structure with an OH group and a positively charged mercury (Hg) bound to an acetate (OAc) group.
- There's an OR group (possibly an alkoxide) and a CH₃ group attached.
2. **Second Structure:**
- Another cyclic structure with an OAc group attached to a positively charged mercury (Hg).
- It includes an OR group and a CH₃ group, with a hydrogen (H) also shown.
3. **Third Structure:**
- A ring structure with a positively charged mercury (Hg) acetate complex.
- OR and CH₃ groups are present, with a hydrogen (H).
4. **Fourth Structure:**
- This shows a cyclic ether with mercury acetate (HgOAc) attached.
- An OR group extends from the carbon adjacent to a CH₃ group.
These structures likely represent intermediates in an organomercury reaction, possibly involving oxymercuration-demercuration or a similar transformation process. The arrows indicate movement of electrons or bonds, typical in reaction mechanisms.
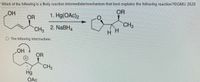
Transcribed Image Text:**Reaction Mechanism and Intermediate Analysis**
**Question:**
Which of the following is a likely reaction intermediate/mechanism that best explains the following reaction? ©GMU 2020
**Chemical Reaction Steps:**
1. **Reactants:**
- A cyclic alkene with a hydroxyl group (OH) and an alkoxy group (OR) attached.
- A methyl (CH₃) group is also attached to the alkene.
2. **Reagents:**
- Step 1: Hg(OAc)₂
- Step 2: NaBH₄
3. **Product:**
- A cyclic ether with an alkoxy group (OR) and an additional methyl group (CH₃) attached to the ring.
**Proposed Intermediate:**
- The mechanism proposes the formation of a mercurinium ion intermediate.
- This intermediate includes a three-membered ring involving the mercury (Hg) atom, which is positively charged.
- The hydroxyl (OH) group coordinates to the mercury atom, while an acetate ion (OAc) is displaced.
- The molecular structure suggests a bridged cyclic ion, showing the stabilization of the positive charge through the formation of the mercurinium ion.
This mechanism accounts for the regioselectivity and stereochemistry observed in the final cyclic ether product.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 11. A material safety data sheet (MSDS) for a chemical is shown below. MSDS H3PO4(aq) Section 9: Physical and Chemical Properties Physical state and appearance: Viscous liquid Odor: Odorless Color: Clear, colorless Boiling point: 158°C Melting point: 21°C Specific gravity: 1.685 at 25°C Which of these is the appropriate name for H3PO4 (aq)? A. Trihydrogen phosphite B. Phosphoric acid C. Phosphorous hydroxide D. Phosphorous acidarrow_forwardOrder these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each species. Select 1 next to the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next higher pH, and so on. Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the pH of pure water to the pH of the other solutions. Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases. relative pH of 0.1 M aqueous solution species H20 4 H,PO4 5 HNO2 (Choose one) ▼ NO, 7 ОН (Choose one) ▼ HF |(Choose one) ▼arrow_forwardCan someone help with this multistep sytheisis and explain why they got their answers.arrow_forward
- 1. NaCN H. 2. H20arrow_forwardBl pure substances, physically combined. Good examples of a solid mixture would be a salad or a bowl of trail mix. Solutions are mixtures too. A spoon full of sugar dissolved in water is an example of both a mixture and a solution. Because mixtures are physical combinations, they are easily separated. Here is a list of mixtures. Match the best method to separate the parts of each mixture. Boil the water. Collect the solid. ITEM BANK: Move to Top drag and drop answer here Dissolve salt in water. Pour through filter paper and collect the sand. Gravel and rocks drag and drop answer here Sand and gravel Remove iron filings with magnet. drag and drop answer here Sand and iron filings Sand and salt Use a screen to sift and separate the big pieces from smaller pieces. drag and drop answer here Sand and wood shavings drag and drop answer here Sugar water Pick out the bigger particles. drag and drop answer here Add water and float one type of particle. 53°F A O O a search 立arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY