
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
Which Ami no acids can be found in the following peptide?
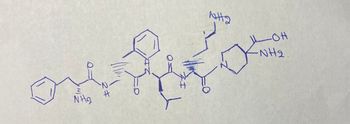
Transcribed Image Text:NH₂
2+
-OH
-NH2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Similar questions
- you have isolated the following peptide: His-Ser-Arg- Ala-Glu- Leu- Pro- Gly Calculate the approximate charge of the peptide at Ph 1, 3, 5, 8, 11, 14 AND what is the PI of this peptide?arrow_forwardA decapeptide was analyzed and the following information obtained. Determine the primary structure of the peptide. Edman's Degradation: PTH-Ala Trypsin produces 3 peptides of the following composition: 1. Gly, His 2. Ala, Leu, Lys, Val 3. Arg (2), Phe, Pro Chymotrypsin produces 2 peptides of the following composition: 1. Ala, Leu, Lys, Phe, Val 2. Arg (2), Gly, His, Pro Elastase produces 1 amino acids and 3 peptides 1. Ala 2. Leu, Val 3. Arg (2), Lys, Phe, Pro, Gly, His Arg Gly His Leu Phe Pro Valarrow_forwardDraw the structure of VEDMN at pH 12. What is the isoelectric point of the peptide (show how you obtain your answer)?arrow_forward
- What are the charges of the following amino acids peptides at ph 14? 1. GLAVV 2. RRKKQarrow_forwardWhat is the net charge at pH 7 on a peptide with the following sequence? Gly-Ala-Lys-Phe-Asp-Met-Val-Pro-Arg-Ala-Leuarrow_forwardDraw the structure of the PTH derivative you would obtain by Edman degredation of the peptide: ALPF.arrow_forward
- What is the isoelectric point of the following peptide molecule? Asp-His-Glu-Gly-Lys-Tyr-Leu-Phe-Arg-Ser-Cys Relevant pKa values are; 2.2, 3.8, 4.3, 6.0, 8.3, 9.8, 10.1, 10.5, 12.5 and 13.1 3.0 9.1 7.2 5.2 4.1 ONLY one correct answerarrow_forwardDiscuss the reaction conditions you would need to carry out the PEGylation reaction of this peptide and explain how the pH affects the selectivity of these reactions.arrow_forwardAt what pH does peptide the His-Arg have a net charge of +1.7?arrow_forward
- Which of the following peptides is more likely to take up an α-helical structure, and why?(a) LKAENDEAARAMSEA(b) CRAGGFPWDQPGTSNarrow_forwardGive some instances of basic peptides that are physiologically active.arrow_forwardWhat is the isoelectric point for the peptide ACDEF at pH 12? Draw the structure.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON