
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
Consider a peptide with the sequence Ala-Glu-Arg-Leu. Assume the ionizable groups have the pKa values listed in Table 2.1 of your text.
(a) Draw the predominant ionic form of the peptide at pH 7.4.
(b) Determine the net charge of the predominant form of the peptide at pH 7.4.
(c) Calculate the pI of the peptide.
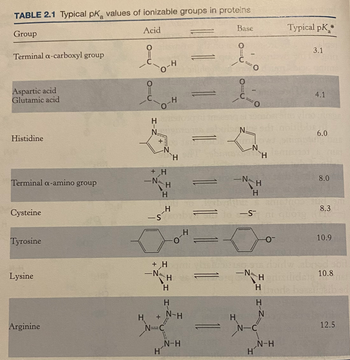
Transcribed Image Text:TABLE 2.1 Typical pK values of ionizable groups in proteins
Group
Terminal a-carboxyl group
Aspartic acid
Glutamic acid
Histidine
Terminal a-amino group
Cysteine
Tyrosine
Lysine
Arginine
Acid
H-N
H
N.
-N
O-H
O
+
+ H
-N
-S
-N
D...
H
H
+ H
H
H
Ilm.
N=C
H
H
H
H
H
+ N-H
O
N-H
H
Base
O
H
C=O
O
1
<=0
N.
-N
-NH
H
-S
H
N-C
H
-O
sors doidw
-N
-Homicilidsta
H
Typical pK *
N-H
3.1
4.1
6.0
8.0
8.3
10.9
10.8
12.5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Similar questions
- Amino acid structure For any ionizable group, indicate the pka value and draw the structure that would be expected at the pH inside the cell (~7.4).arrow_forwardBased on the following image, what is the N-terminal AA residue, C-terminal AA residue? What is also the sequence of peptide 2 and peptide 3? Also identify the overall amino acid sequencearrow_forwardPatients suffering from sickle cell anemia have a mutation in the gene that codes for one of the hemoglobin chains, in which a single glutamate is replaced by a valine. Propose an explanation for why this substitution has such a striking effect on protein structure and function with explanation. Suggest two other amino acids that would be less likely to cause such serious impairment of hemoglobin function if each was substituted for glutamate. Briefly explain your answer.arrow_forward
- At which pH would the peptide with the amino acid sequence 'KEYHR' be found in an IPG (Immobilized pH gradient) strip after successful isoelectric focusing. Show calculation. lonizable groups Alpha amine group of K Side chain group of K Side chain group of E Side chain group of Y Side chain group of H Side chain group of R Alpha carboxylic acid group of R pka 8.95 10.53 4.25 10.07 6 12.48 2.17arrow_forwardWhat is the net charge on the peptide RHTLE at pH 12.5?arrow_forwardThe amino acid arginine ionizes according to the following scheme: (a) Calculate the isoelectric point of arginine. You can neglect contributions from form I. Why? (b) Calculate the average charge on arginine when pH = 9.20. (c) Is the value of average charge you calculated in part b reasonable, given the pI you calculated in part a? Explain your answerarrow_forward
- Consider the peptide Trp-Arg-Glu-Cys-Gly-Tyr. For the drawings requested below, please show them in zig-zag style, from amino to carboxy terminus, with correct stereochemistry Draw the predominant form at pH = 2 Draw the predominant form at pH = 5 Draw the predominant form at pH = 7 Draw the predominant form at pH = 12arrow_forwardPlease draw titration curves for peptide HFDTYA and HEKDGQ, and calculate the pl for each. Usethis information to explain why the peptide HFDTYA has low solubility and HEKDGQ has a highersolubility in bulk water at pH = 7.arrow_forwardConsider a short peptide with the sequence M-C-Q-L-Y-P-E-D-K. List the ionizable groups in this peptide, and the net charge of the whole peptide at pH a) 1.5, b) 7.0, and c)12.0.arrow_forward
- There is parts A-D for the picture provided. A) A peptide has the sequence, Arg-Cys-His-Tyr-Glu-Asn-Lys-Asp. What is the net charge at pH 1? Choices: -5 -4 -3 -2 -1 0 +1 +2 +3 +4 +5 B) A peptide has the sequence Arg-Cys-His-Tyr-Glu-Asn-Lys-Asp. What is the net charge at pH 5? Choices: -5 -4 -3 -2 -1 0 +1 +2 +3 +4 +5 C) Same Peptide sequence, What is the net charge at pH8.5, (N-terminus is pronated) Choices: -5 -4 -3 -2 -1 0 +1 +2 +3 +4 +5 D) A peptide has the sequence, Arg-Cys-His-Tyr-Glu-Asn-Lys-Asp. What is the net charge at pH 13? Choices: -5 -4 -3 -2 -1 0 +1 +2 +3 +4 +5 Thank You!arrow_forward1) Indicate the total charge of the peptide at pH 7.0, 9.0, and 11.0 2) Determine the isoelectric point (pI) for this peptide. Show the relevant pKa’s and your calculation. 3) What is the fraction of the peptide that has the N-terminal amino group deprotonated at pH 9.arrow_forwardcalculate the net charge of your peptide at I) pH 2.0; ii) pH 6.0 iii) pH 7.0; iv) pH 11.5. show full working and include justification as to how you determined the charge of WEIJIN peptidearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON