
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
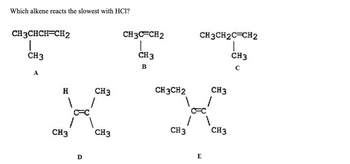
Transcribed Image Text:Which alkene reacts the slowest with HC1?
CH3CHCH=CH2
CH3
A
Н
CH3
D
CH3
CH3
CH3CFCH2
CH3
B
CH3CH2
CH3
CH3CH2C=CH2
|
CH3
с
E
1
CH3
CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Suzuki coupling of aryl iodide A and vinylborane B affords compound C, which is converted to D in the presence of aqueous acid. Identify compounds C and D and draw a stepwise mechanism for the conversion of C to D.arrow_forwardWhat is the predicted product of the reaction shown? NO2 H2 CH=CH2 Ni NH2 NH2 NO2 CH=CH2 CH2-CH3 CH2-CH3 II ZON ZON CH=CH2 CH=CH2 IV Varrow_forwardDraw the products of these reactions from a-earrow_forward
- Rank the following alkyl halides in order of their expected SN1reactivity. CH3CH2Br H2C=CHCH(Br)CH3 H2C=CHBr CH3CH (Br)CH3arrow_forwardRank the molecules A to D shown below by decreasing reactivity towards lodo cyclohexane. H3C-CH,OH F,C-CH,OH H,C-CH,NH, H3C-N-CH, A в D O 1.C>D>A>B O 2.B> A> C> D O 3. A> C> B > D O 4. A> B>D>Carrow_forwardC341 5.) Draw a step-by-step mechanism for the reaction of 1-methyl-1-cyclopentene with the following: a.) hydrogen bromide (i.e., hydrohalgenation) H-Br b.) Br2 in CH2Cl2 (i.e., halogenation) Br-Br c.) Br2 in ethanol (i.e., halohydrin) Br Br OH Chapter 8: Alkenesarrow_forward
- Identify the best reagents to convert 1-hexyne into 2-bromo-1-hexene. O xs Br2, CCl4 1 equiv HBr, ROOR xs HBr O 1 equiv HBr O 1 equiv. Br2, CCl4arrow_forwardPredict the products of each reaction below. Indicate regiochemistry and stereochemistry when relevant. CH3 X™ CH3 H₂C H H CH3 H₂ Pd CHC13 KOHarrow_forwardWhat would be the FINAL result of the reaction(s) shown below? H₂O CH3CH2-CEC-H ? cat H2SO4 cat HgSO4 H OH `c = c' CH3CH2CH2-C-H H с D HO H C=C CH3CH2 H CH3CH2-C-CH3 CH3CH2 A B D C A Barrow_forward
- What set of reagents can best accomplish the following transformation? NH NH `NH ? 90% -10% A) PCC in CH2C2 B) AICI3 C) HCI in Et,O D) H2SO4, HNO3 E) Cl2, FeCl3 O B O A O Earrow_forwardThe following 3° carbocation is more stable than a typical 3° carbocation because it is CH3 A stabliized.arrow_forwardWhat is the expected product of the reaction below: CH3 + CH3-C-Br + Na : CCH CH3 CH3. CH3 CH3 CH3 | CH2 H-C=C-H CH3--C=C-H CH3 CH2 =C-CH3 and CHS II || CH3 H-C=C-CH2-CH-CH3 IVarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY