
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
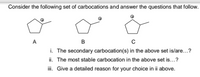
Transcribed Image Text:Consider the following set of carbocations and answer the questions that follow.
A
в
i. The secondary carbocation(s) in the above set is/are...?
ii. The most stable carbocation in the above set is...?
i. Give a detailed reason for your choice in ii above.
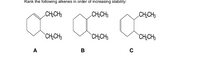
Transcribed Image Text:Rank the following alkenes in order of increasing stability:
CH;CH3
CH2CH3
CH;CH3
CH,CH3
CH,CH3
CH,CH3
A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. Complete the following reaction wheel providing structures or reagents where needed. Show stereochemistry where necessary. d e j 1). BH3.THF 2). H₂O₂, OH* 1). Na 2). CH3CH₂Br 1). CH3MgBr 2). H3O+ a 2). H3O+ с j 1 k 1). CH3CH₂MgBr CrO3, H+ TSCI Pyridine 1. Br₂, H₂O h Mal H*/heat OHarrow_forwardPlease help with part E, F, G:arrow_forwardCan anyone explain how to solve this?arrow_forward
- Please don't provide handwritten solution ....arrow_forwardRank the carbocations below in order of increasing stability (least stable = 1; most stable = 3). Place the number corresponding to the carbocation's relative stability in the blank below the structure. Pathway B CH3 CH3 I (CH3)2C-CH-CH₂ + CH₂ Below are all the chemical structures and intermediates involved in a reaction. On the structures provided, show all electron flow using the arrow formalism for the complete stepwise mechanism. Pathway A (CH3)3CCH=CH₂2 H-Cl (CH3)3CCH-CH3 :Cl CH3 CH3 (CH₂)2C-CH-CH₂ (CH3)3CCHCH3 Cl A Cl I (CH3)₂ CCHCH; T CH3 Barrow_forwardProvide the systematic or common names of the compounds shown below in the blanks given, including stereochemistry (i.e., E, Z, R, S, etc.) if necessary. но H. ČI Name Name Namearrow_forward
- Draw a structural formula for the more stable carbocation intermediate formed in the reaction shown. CH3CH2 CH₂CH₂CH3 H H + HI • You do not have to consider stereochemistry. • Do not include anionic counter-ions, e.g., I, in your answer. • For cases in which carbocations of the same or similar stability are expected, draw all of the structures. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate structures with + signs from the drop-down me menu. ChemDoodleⓇ [ ] در > Previous Ne:arrow_forwardIdentify ALL products expected from this reaction. Bonus question (for class): Draw the products expected if water were added instead of CH2Cl2 Br2 CH2Cl2 Br Br Br Br Br Br.. Br Br A B C Darrow_forwardDraw the intermediate product, be sure to include formal charges. Do not include lone pair electrons. :O: OCH3 1,4-addition of the Ph2S sulfur ylide to the B carbon edit structure ... a sulfur ylidearrow_forward
- How many and what type of products will form if the following molecule undergoes E1? H Br choose your answer... V total product(s) form. choose your answer... V will be optically active.arrow_forwardIn parts 1 and 2 draw the two organic products of this reaction, showing any nonzero formal charges. Then, in part 3 answer the question regarding purification of the reaction mixture. 1. Draw the product with the higher molecular weight here: 2. Draw the product with the lower molecular weight here:arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY