
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
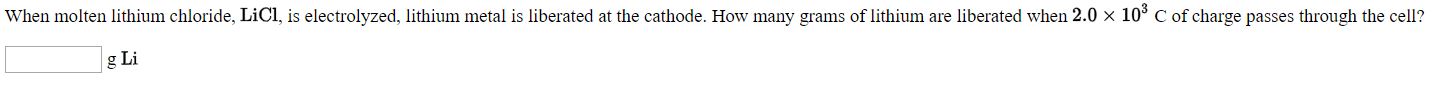
Transcribed Image Text:When molten lithium chloride, LICI, is electrolyzed, lithium metal is liberated at the cathode. How many grams of lithium are liberated when 2.0 x 10° C of charge passes through the cell
g Li
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A certain metal è forms a soluble sulfate salt ₂(so). Suppose the left half cell of a galvanic cell apparatus is filled with a 5.00 M solution of M₂(SO4)3 and the right half cell with a 250. mM solution of the same substance. Electrodes made of M are dipped into both solutions and a voltmeter is connected between them. The temperature of the apparatus is held constant at 35.0 °C. Which electrode will be positive? O left Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits. O right What voltage will the voltmeter show? Assume its positive lead is connected to the positive electrode. 0 x10 Xarrow_forwardA certain metal M forms a soluble sulfate salt M₂SO4. Suppose the left half cell of a galvanic cell apparatus is filled with a 2.00 M solution of M₂ SO4 and the right half cell with a 10.0 mM solution of the same substance. Electrodes made of M are dipped into both solutions and a voltmeter is connected between them. The temperature of the apparatus is held constant at 25.0 °C. left Which electrode will be positive? x10 right X Ś ? What voltage will the voltmeter show? Assume its positive lead is connected to the positive electrode. 0 Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.arrow_forwardThe chemical reaction that causes iron to corrode in air is given by: 4Fe+3O2→2Fe2O3 in which at 298 K, ΔHorxn = −1684 kJ and ΔSorxn = −543.7 J/K At what temperature Teq do the forward and reverse corrosion reactions occur in equilibrium?arrow_forward
- Questions 20 and 21 refer to the following information. Galvanic Cell Cell Diagram E°cell (Volts) Mg | Mg2(1.0 M) || Pb*2(1.0 M) | Pb 1 2.24 Zn | Zn(1.0 M) || Pb*2(1.0 M) | Pb 2 0.63 Mg | Mg*(1.0 M) || Zn*2(1.0 M) | Zn 3 The chemical reaction occurring in first galvanic cell is: Mg + Pb*2 → Mg+2 + Pb 21. If the concentration of Mg+2 is changed from 1.0 M to 3.0 M in the galvanic cells above, what will happen to the observed cell voltages in galvanic cells 1 and 3? (A) The voltage in both galvanic cells 1 and 3 will increase. (B) The voltage will decrease in both galvanic cells 1 and 3. (C) The voltage in galvanic cell 1 will increase and will decrease in galvanic cell 3 (D) The voltage in galvanic cell 3 will increase and will decrease in galvanic cell 1. 22. Consider a voltaic cell based on these half-cells. Ag (aq) +e → Ag/s) Cd (aq) + 2e → Cd¢s) E* = +0.80 V E = -0.40 V Identify the anode and give the voltage of this cell under standard conditions. (A) Ag; Ecell = 0.40 V (B) Ag;…arrow_forward2 A certain metal M forms a soluble nitrate salt M(NO3)₂. Suppose the left half cell of a galvanic cell apparatus is filled with a 1.00 M solution of M(NO3), and the right half cell with a 100. mM solution of the same substance. Electrodes made of M are dipped into both solutions and a voltmeter is connected between them. The temperature of the apparatus is held constant at 35.0 °C. left x10 Which electrode will be positive? right X ? What voltage will the voltmeter show? Assume its positive lead is connected to the positive electrode. 0 Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.arrow_forwardSilver metal can be dissolved in acidic solutions via a redox reaction with generation of hydrogen gas. Which of the following is the correct cell notation for the reaction that will occur?arrow_forward
- Which of the following is the correct shorthand notation for the overall cell reaction given below. Mg(s) + Cu2+(aq) → Mg2+(aq) + Cu(s) Select one: a. Mg(s) | Mg2+(aq) || Cu2+(aq) | Cu(s) b. Cu(s) | Mg2+(aq) || Cu2+(aq) | Mg(s) c. Mg(s) | Cu2+(aq) || Mg2+(aq) | Cu(s) d. Cu(s) | Cu2+(aq) || Mg2+(aq) | Mg(s)arrow_forwardA certain metal M forms a soluble sulfate salt M, SO4. Suppose the left half cell of a galvanic cell apparatus is filled with a 250. mM solution of M,SO, and the right half cell with a 2.50 M solution of the same substance. Electrodes made of M are dipped into both solutions and a voltmeter is connected between them. The temperature of the apparatus is held constant at 35.0 °C. left Which electrode will be positive? Ox10 right ? What voltage will the voltmeter show? Assume its positive lead is connected to the positive electrode. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.arrow_forwardA certain metal M forms a soluble nitrate salt M(NO3)3. Suppose the left half cell of a galvanic cell apparatus is filled with a 2.00 M solution of M(NO3)3 and the right half cell with a 10.0 mM solution of the same substance. Electrodes made of M are dipped into both solutions and a voltmeter is connected between them. The temperature of the apparatus is held constant at 20.0 °C. Which electrode will be positive? What voltage will the voltmeter show? Assume its positive lead is connected to the positive electrode. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits. 0 0 ○ left ☐ x10 O right ×arrow_forward
- Consider the electrochemical cell diagram shown below. As you observe the reaction in the cell, you notice that the tin electrode seems to be disappearing while there are deposits forming on the silver electrode. Which of the following is a correct statement? Sn KNO3(aq) Ag IM Sn (NO3)2 IM AgNO3 O The tin electrode is the cathode and the silver electrode is the anode. O Electrons are flowing from the silver electrode to the tin electrode. O Potassium ions are flowing through the salt bridge to the silver solution. O The half-reaction occurring at the tin electrode is Sn4+ + 2e → Sn2+arrow_forwardThe oxidation of methanol, as described by the equation below, has a ΔG° value of -937.9 kJ/mol. What is the standard cell potential (in V) for a methanol fuel cell? 2 CH3OH (l) + 3 O2 (g) → 2 CO2 (g) + 4 H2O (l)arrow_forwardA certain metal M forms a soluble nitrate salt M(NO3)2. Suppose the left half cell of a galvanic cell apparatus is filled with a 2.50 mM solution of M (NO3)2 and the right half cell with a 5.00 M solution of the same substance. Electrodes made of M are dipped into both solutions and a voltmeter is connected between them. The temperature of the apparatus is held constant at 20.0 °C. Which electrode will be positive? What voltage will the voltmeter show? Assume its positive lead is connected to the positive electrode. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits. Π left x10 rightarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY