
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
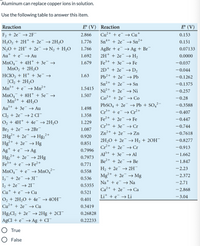
Transcribed Image Text:Aluminum can replace copper ions in solution.
Use the following table to answer this item.
Reaction
E° (V) Reaction
E (V)
F2 + 2e¯ –→ 2F¯
2.866
Cu²+ + e¯
→ Cu+
0.153
4+
2+
H2O2 + 2H* + 2e¯ → 2H2O
1.776
Sn
+ 2e¯ → Sn“
0.151
AgBr + e
2H* + 2e¯ –→ H2
N20 + 2H* + 2e¯ → N2 + H,O
1.766
→ Ag + Br
0.07133
Au+ + e¯ –→ Au
1.692
0.0000
MnO4 + 4H† + 3e¯ →
MnO2 + 2H3O
1.679
Fe3+ + 3e¯ → Fe
-0.037
2D* + 2e¯ –→ D2
-0.044
HСIO, + H* + Зе —
Cl2 + 2H,O
Mn+ + e¯ → Mn²+
1.63
Pb2+ + 2e¯ → Pb
-0.1262
Sn²+ + 2e¯ → Sn
-0.1375
1.5415
Ni?+ + 2e¯ → Ni
-0.257
MnO4 + 8H* + 5e°
Mn²+ + 4H2O
1.507
Co²+ + 2e¯ → Co
-0.28
PBSO4 + 2e¯ → Pb + SO4²¬
Cr+ + e¯ → Cr²+
-0.3588
Au³+ + 3e¯ → Au
1.498
-0.407
Cl2 + 2e¯ → 2 Cl¯
O2 + 4H† + 4e
1.358
Fe2+ + 2e¯ → Fe
-0.447
→ 2H2O
1.229
Cr³* + 3e¯ → Cr
-0.744
Br2 + 2e
2H9²+ + 2e¯ → Hg2²
Hg²+ + 2e
Ag* + e¯ → Ag
Hg,
→ 2Br
1.087
Zn²+ + 2e¯ → Zn
-0.7618
2+
0.920
2H2O + 2e¯ → H2 + 20H-
Cr2+ + 2e¯ → Cr
-0.8277
→ Hg
0.851
-0.913
0.7996
Al³+ + 3e¯ –→ Al
-1.662
2+
+ 2e
→ 2Hg
0.7973
Be2+ + 2e¯ → Be
-1.847
Fe+ + e → Fe2+
0.771
Н, + 2е — 2H
-2.23
MnO4 + e¯ → MnO4²-
0.558
Mg²+ + 2e¯ –→ Mg
- 2.372
I3 + 2e¯ -→ 3I¯
0.536
Na* + e –→ Na
-2.71
I2 + 2e
→ 21-
0.5355
Ca2+ + 2e¯ → Ca
- 2.868
Cu* + e¯ –→ Cu
0.521
Lit + e → Li
- 3.04
O2 + 2H2O + 4e¯ → 40H-
Cu²+ + 2e
0.401
→ Cu
0.3419
Hg,Cl, + 2e¯ → 2Hg + 2CI-
0.26828
AgCl + e¯ → Ag + Cl¯
0.22233
True
O False
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Balance the following redox reaction in basic solution. H2(g)+Cl₂(9) H2O(l)+Cl (aq) H2(g) + C₁₂ (g) → H₂O(l) + Cl¯ (aq) - ローロ ☑arrow_forwardBalance the following half-reactions by adding the appropriate number of electrons (e-). Then, classify each reaction as an oxidation or reduction half-reaction. Note that for each of the four reactions, one of the gray boxes will be left blank and the other will be filled with electron(s). Use the symbol e- to represent an electron. Part 4 + 12(s) + 6H2O(1) > 2I0-3 + 12H+(aq) + --arrow_forward+ 1.14 V TI Pt Cr3+ (1 M) Cr2 (1 M) T13+ (1 M) T13+ (aq) + 3e-→ TI (s) Cr2+ (aq) → Cr3+ (aq) + e- What happens to the thallium electrode as the redox reaction progresses? O it gains mass as thallium metal is plated onto the electrode O it loses mass as the thallium metal is consumed by the reaction O it spontaneously ignites and burns O the thallium electrode is inert and unaffected by the redox reaction O a thick layer of thallium salt develops on the clectrode DELLarrow_forward
- Balance the following reaction in acidic solution. Fe?+ (ag) + MnO, (aq) → Fe+ (ag) + Mn²+(aq) Fill in the coefficients and substances for the balanced overall equation. Note: Do NOT leave any coeffient fields empty. If the coefficient is 1, choose "1" as opposed to leaving it blank. Fe2+ + MnO,¯ + 4 Fe3+ + Mn2+ +arrow_forwardConsider the redox reaction shown below: IO3-1(aq) + 5 I-1 (aq) + 6 H+1 (aq) → 3 H2O (l) + 3 I2 (aq) What is the reducing agent?arrow_forward10. Balance the following redox reaction using the half-reaction method and tell how many electrons are transferred in the reaction. Ag(s) + CN (aq) +O2 (g) → Ag(CN), + H2O (BASIC medium) HINT: CN- is neither oxidized nor reducedarrow_forward
- The following skeletal oxidation-reduction reaction occurs under acidic conditions. Write the balanced OXIDATION half reaction. Co2+ + NO Co + NO3-arrow_forwardThe amount of lactic acid, HC3H5O3, produced in a sample of muscle tissue was analyzed by reaction with hydroxide ion. Hydroxide ion was produced in the sample mixture by electrolysis. The cathode reaction is given below. 2 H2O(l) + 2 e− → H2(g) + 2 OH− (aq) Hydroxide ion reacts with lactic acid as soon as it is produced. The endpoint of the reaction is detected with an acid-base indicator. It required 104 s for a current of 13.1 mA to reach the endpoint. How many grams of lactic acid (a monoprotic acid) were present in the sample?arrow_forwardIn the balanced redox reaction: 2 Cu(s) + S(s) > Cu 2S(s); how many electrons are gained or lost by each copper atom?arrow_forward
- In the following redox reaction identify the element undergoing oxidation and the element undergoing reduction. 2NO(g)+O2(g)⟶2NO2(g)arrow_forwardPredict which species will be oxidized and which will be reduced in a molten salt mixture that contains CdI2, NaOH, and AlCI3. Cd2+(aq) + 2e → Cd(s) Eored = – 0.40 V I2(s) + 2e → 2 I–(aq) Eored = + 0.54 V Na+(aq) + 1e → Na(s) Eored = – 2.71 V Cl2(g) + 2e → 2 Cl–(aq) Eored = + 1.36 V Al3+(aq) + 3e → Al(s) Eored = – 1.66 V O2(g) + 2 H2O(l) + 4e → 4 OH–(aq) Eored = + 0.40 V which is oxidized which is reducedarrow_forward/variants/1189727/take/9/ ☆ 88 MULTIPLE CHOICE Question 10 Which half-reaction correctly represents oxidation? Fe(s) → Fe +2 A Fe(s) B Fe +2 (aq) + 2e¯ (aq) → Fe(s) + 2e- + 2e → Fe+2 (ag) D Fe +2 (aq) + 2e¯ → Fe(s)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY