
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
how can i plot this data for concentration with depth?
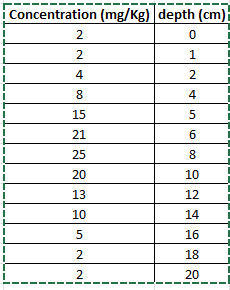
Transcribed Image Text:Concentration (mg/Kg) depth (cm) -
2
0
2
1
4
2
8
4
15
5
21
6
25
00
8
20
10
13
12
10
14
5
16
2
18
2
20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Similar questions
- (This question and the next 2 questions are breakdowns of the same question) A student John wanted to measure the molar mass of an unknown material (UM) via the boiling point change of a solvent. He chose a solvent, ethanol, that can dissolve the material and sugar. He wanted to use sugar (MW = 342.3 g/mol) as a reference sample to get an ebullioscopic constant for his system to reduce the effect of other measuring errors such as pressure, measuring errors, and impurity of the samples. He made 10 solutions each contain 100 g of ethanol and various amont of solute as list in the table. He fits the two sets of data and obtain the molar weight of the unknown materials. For this question answer the fitted boiling point of pure ethanol: ____ K. 4 sig. fig. Thre rest ? marks in the tale will be answered in the next two questions. Copy the data into Excel and use trendline to fit or calculate the average values for this and the next 2 questions. Sample # Sugar (g) B.P. (K) Sample # UM…arrow_forwardHint: You won't be able to tell exactly when the substance melts. You don't need to have exact measurements, just try to get an approximate value. Row Name melting temp °C variable 1 CuCI 2 Sucrose KI ... 4. lodine ... ...arrow_forwardDiscuss the relationship between chemical potential and fugacity. (Discuss briefly in 300 words)arrow_forward
- a)Graph the distance migrated versus concentration for xylene cyanol series on the semi log graph. b)How would you describe this relationship between the concentration of the dye and rate of diffusion. c)When compared to the diffusion of the same concentration of pure c)xylene chabolista does the presence of a second source in the mixture affect the diffusion rate of xylene cyanol?arrow_forwardThe liquid-liquid extraction technique takes advantage of the solubility difference of a given compound in two immiscible solvents. Group of answer choices True Falsearrow_forwardThe distance travelled by the compound depends on how soluble it is in the chosen solvent. True False 2021-11...50.03 PM Screen Shot 0031.11...50.12 PMarrow_forward
- List the three different cases that we studied for comparison of means, and write the equations used in each case.arrow_forwardGiven that ln(A1) = ln(A0) – kt1 ……….. (i) ln(A2) = ln(A0) – kt2 ……….. (ii) Where A1 = A0 - x1 A2 = A0 - x2 Y = x2 – x1 Prove that x(1)={Ao-[y/(1-e^-k(t2-t1)]} Using SOUND, BALANCED NUCLEAR EQUATION/REACTION AND PRINCIPLE ONLY, explain “How does KI work to help mitigate the effect of exposure to radiation?” http://www.thestar.com/news/article/954546--radiation-fears-spark-run-on-iodide-pills ® ® ® “the SOURCE OF HEAT that resulted in the melt-down at the Fukushima-nuclear-reactor?” [Actual balanced nuclear equations showing heat generated or absence of certain things required for full point]…arrow_forwardNeed help with this question. Thank you :)arrow_forward
- 175.0 mL of 0.10 M C2H5NH2 with 285.0 mL of 0.20 M C2 H5NH3 Cl Express your answer using two decimal places. ΑΣφ ...... pHarrow_forwardYour measurements indicate that the concentration at valve V7 is 2.04 ppm, while the concentration at thetop of the column is 3.06 ppm. Calculate the normalized concentration at V7. Report using four decimalfigures to an accuracy of ± 0.0001.arrow_forwardExpress the following points given in Cartesian coordinates in terms of spherical coordinates. (x, y, z): (1, 0, 0); (0, 1, 0); (0,0,1); (0,0,-1)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY