
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
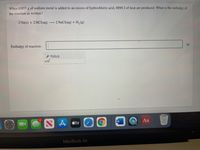
Transcribed Image Text:When 0.855 g of sodium metal is added to an excess of hydrochloric acid, 8890 J of heat are produced. What is the enthalpy of
the reaction as written?
2 Na(s) + 2 HCl(aq)
2 NaCl(aq) + H,(g)
>
kJ
Enthalpy of reaction:
TOOLS
x10
Aa
étv
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following reaction. CH3OH(g) CO(g) + 2 H2(g) H = +90.7 kJ (a) Is the reaction exothermic or endothermic? exothermic endothermic (b) Calculate the amount of heat transferred when 55.0 g of CH3OH(g) are decomposed by this reaction at constant pressure.H = kJ(c) If the enthalpy change is 11.0 kJ, how many grams of hydrogen gas are produced? g(d) How many kilojoules of heat are released when 12.0 g of CO(g) reacts completely with H2(g) to form CH3OH(g) at constant pressure?H = kJ(e) Calculate E when 450.0 g of CH3OH(g) completely reacts at a constant temperature of 300 K and constant pressure of 0.95 atm. R = 8.314 J/mol*K and R = 0.08206 atm*L/mol*Karrow_forwardConsider the following reaction. CH3OH(g) CO(g) + 2 H2(g) H = +90.7 kJ (a) Is the reaction exothermic or endothermic? endothermicexothermic (b) Calculate the amount of heat transferred when 40.0 g of CH3OH(g) are decomposed by this reaction at constant pressure.H = kJ(c) If the enthalpy change is 11.0 kJ, how many grams of hydrogen gas are produced? g(d) How many kilojoules of heat are released when 13.5 g of CO(g) reacts completely with H2(g) to form CH3OH(g) at constant pressure?H = kJ(e) Calculate E when 360.0 g of CH3OH(g) completely reacts at a constant temperature of 300 K and constant pressure of 0.95 atm. R = 8.314 J/mol*K and R = 0.08206 atm*L/mol*K kJ HopHelpCh5N1arrow_forwardWhen 0.638 of sodium metal is added to an excess of hydrochloric acid, 6630j of heat are produced. What is the enthalpy of the reaction as written? 2Na(s)+2HCl(aq)⟶2NaCl(aq)+H2(g) Enthalpy of reaction:arrow_forward
- A student runs two experiments with a constant-volume "bomb" calorimeter containing 1300. g of water (see sketch at right). thermometer stirrer First, a 6.000 g tablet of benzoic acid (C,H,C0,H) is put into the "bomb" and burned completely in an excess of water oxygen. (Benzoic acid is known to have a heat of combustion of 26.454 kJ/g.) The temperature of the water is insulation observed to rise from 13.00 °C to 40.13 °C over a time of 10.0 minutes. Next, 5.200 g of ethane (C,H) are put into the "bomb" and similarly completely burned in an excess of oxygen. This time the temperature of the water rises from 13.00 °C to 53.96 °C. chemical reaction "bomb" Use this information, and any other information you need from the ALEKS Data resource, to answer the questions below about this reaction: A "bomb" calorimeter. 2C,H,(g) + 70,(g) 4CO,(g) + 6 H,0 (g) Be sure any of your answers that are calculated from measured data are rounded to the correct number of significant digits. Note for advanced…arrow_forwardDetermine the enthalpy of reaction (DHxn) in kJ/ mol H20 formed if an acid/base neutralization if 13.6 mL of 1.205 M NaOH is reacted with 25.0 mL of 1.108 M HCI causing an increase in the calorimeter temperature of 6.85 °C. Use 3.93 J/g°C for the specific heat capacity of the final solution and the density for the final solution is 1.02 g/mL.arrow_forwardWhen ammonia reacts with dinitrogen oxide gas (ΔHf° = 82.05 kJ/mol), liquid water and nitrogen gas are formed. How much heat (in kJ) is liberated or absorbed by the reaction that produces 365 mL of nitrogen gas at 25°C and 743 mm Hg? ΔHf° (kJ/mol) NH3(g) –46.1 H2O(l) –285.8 N2(g) 0 N2O(g) 82.05arrow_forward
- When ammonia is oxidized it produces nitrogen monoxide and heat: 4NH3(g)+ 5O2(g)--> 4NO(g) +6H2O(g) "Triangle symbol"H degrees= -906kJ (a) Is the reaction endothermic or exothermic? Explain your answer. (b) If the reaction is carried out in a bomb calorimeter, will the temperature of the calorimeter increase or decrease? Explain your answer. (c) Is the internal energy of the products or reactants higher? Explain your answer.arrow_forwardSulfur dioxide, SO2(g), can react with oxygen to produce sulfur trioxide, SO3(g), by the reaction 2 SO2(g) + O2(g) → 2 SO3(g) The standard enthalpies of formation for SO2(g) and SO3(g) are AH; [SO2(g)] = -296.8 kJ/mol AH; [SO3(g)] = -395.7 kJ/mol Calculate the amount of energy in the form of heat that is produced when a volume of 2.25 L of SO2(g) is converted to 2.25 L gas behavior. of SO3(g) according to this process at a constant pressure and temperature of 1.00 bar and 25.0 °C. Assume ideal AH= kJarrow_forwardA student runs two experiments with a constant-volume "bomb" calorimeter containing 1100. g of water (see sketch at right). thermometer stirrer First, a 5.500 g tablet of benzoic acid (C,H,CO, H) is put into the "bomb" and burned completely in an excess of water oxygen. (Benzoic acid is known to have a heat of combustion of 26.454 kJ/g.) The temperature of the water is observed insulation to rise from 15.00 °C to 42.56 °C over a time of 10.3 minutes. Next, 5.720 g of acetaldehyde (C2H,O} are put into the "bomb" and similarly completely burned in an excess of oxygen. This time the temperature of the water rises from 15.00 °C to 40.53 °C. chemical reaction "bomb" Use this information, and any other information you need from the ALEKS Data resource, to answer the questions below about this reaction: A "bomb" calorimeter. 2C,H,0(g) + 50, (g) 4CO, (g) + 4H,0 (g) Be sure any of your answers that are calculated from measured data are rounded to the correct number of significant digits. Note…arrow_forward
- 5(a)arrow_forward(1)Consider the reaction: 2A (g) + 3 B (g) → 2 C (g) ΔHrxn = +254.3 kJ What will be the enthalpy change (in kJ) if 0.471 mol B reacts in excess A? (2)Consider the reaction: C (s) + O2 (g) → CO2 (g) ΔHrxn = -393.5 kJ What mass of carbon (in g) must be reacted via this mechanism to release 581.2 kJ of heat?arrow_forward5aarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY