
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
6
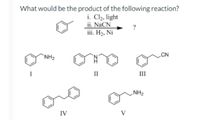
Transcribed Image Text:What would be the product of the following reaction?
i. Cl2, light
ii. NaCN
iii. H2, Ni
`NH2
II
II
NH2
IV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1PrqdNDx-hBYyzKo-xfiM9-Jlq7EAaWe3GBt t3U3jDo/edit ulator O A Land of Permane... it was seconds ago - UA E- EE 2 Al + 3 H,SO, 6. How many grams of hydrogen gas are produced with 0.S8 moles of aluminum sulfate? 3 H, + Al,(SO); 0.88 moles Al (SO), 3 moles H. 6 g H. 0.88 moles Al (SO), 1 mole H.arrow_forwarda) 2 Ob) 1 c) 0 d) 7 e) 5arrow_forward6./Complete hudlear equations beicw. the missing partidro in thC A 23 227 231. १० १) 231arrow_forward
- Im still confused on where you got 3.58, could you please expand upon that?arrow_forwardmistry F20 SE 1. If you have 23.2 grams of gold, what is the volume of the sample? Densities of Some Common Metals Metal Density (g/mL) Copper 8.94 the Aluminum 2.70 Gold 19.3 (A) 0.116 ml (B) 0.832 mL (C) 1.20 ml (D) 8.59 mL DELLarrow_forward8:41 AM Thu Mar 9 Items Back 22-23 Q3 SUM CHEMISTRY ADV Element Hydrogen Oxygen Sulfur A ALEKS - KEH... Molar mass 1.008 g/mol 15.999 g/mol 32.066 g/mol M Sign in to yo... Mach B Technologies Inc. © 2023 - gp.edugence.com ●●● What is the theoretical yield of sulfuric acid? Answer F) 613 g H₂SO4 G) 630 g H₂SO4 H) 651 g H₂SO4 J) 695 g H₂SO4 GP ISD Parents & St... 22-23 Q3 SUM CHEMISTRY ADV e gp.edugenc... SO3 + H₂O → H₂SO4 - x² X ◄▬ 6 500 g of sulfur trioxide react with excess water to produce sulfuric acid H₂SO4 in the reaction represented by the equation below. VPN + S G You find that... × × × × X X 10% X A 5arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY