
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
what is the molecular orbital configurations, number of unpaired electrons, and bond order for B2?
Using the molecular orbital diagram for the molecule in the previous question, what is the HOMO and LUMO for that molecule?
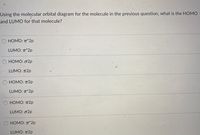
Transcribed Image Text:Using the molecular orbital diagram for the molecule in the previous question, what is the HOMO
and LUMO for that molecule?
HOMO: T*2p
LUMO: T*2p
HOMO: o2p
LUMO: T2p
О НОМО: п2р
LUMO: T*2p
НОМО: п2р
LUMO: o2p
HOMO: T*2p
LUMO: T2p
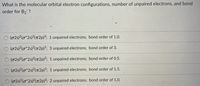
Transcribed Image Text:What is the molecular orbital electron configurations, number of unpaired electrons, and bond
order for B2 ?
O (025)2(0*2s)²(T2p)³; 1 unpaired electrons; bond order of 1.0.
O (02s)2(o*2s)?(72p)3; 3 unpaired electrons; bond order of 3.
(02s)2(o*2s)2(7T2P)³; 1 unpaired electrons; bond order of 0.5.
O (02s)2(o*2s)2(T2p)³; 1 unpaired electrons; bond order of 1.5.
(02s)2(o*25)²(T2p)2; 2 unpaired electrons; bond order of 1.0.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the molecular orbital diagram for the following molecule. Please state any assumptions made when making the MO diagram. Also, draw each of the bonding molecular orbitals. Molecule: MgSarrow_forward15. How many hybrid orbitals do we use to describe each molecule? Enter in integer form! -BrCNarrow_forward2. In the molecular orbital (MO) scheme for frontier orbitals of benzene, answer the following: a) how many of the carbons in benzene are sp²/p hybridized? b) how many p-n electrons does this system have? c) how many T,7* MOs can be constructed from the carbon p-orbitals? d) labeling these yn (n= 1 to ....) increasing in energy, how many vertical nodes do the following MOs have ? Y2,43 ¥4,Y5 e) on the hexagonal skeleton to right, draw the molecular orbital for y3, or y4. Are these MOs bonding, non-bonding or antibonding? Clearly show/tell why. f) in benzene, what orbital(s) is/are the HOMO? yn = g) what is the (value of the) aromatic stabilization energy for benzene? h) is benzene more or less stable than 1,3,5-cyclohexatriene? more less i) in two words, what is the structure of benzene? the LUMO? yn = kJ/mol (circle one)arrow_forward
- Study the following sketch of a molecular orbital (MO) in a homonuclear diatomic molecule. This MO was formed by combining one 3s atomic orbital from each atom. The dark dots in this sketch are the nuclei. Now use the sketch to complete the table below. Write the symbol for this MO. Is this a bonding or antibonding MO? bonding What is the energy of this MO, compared to the energy of a 3s orbital on one of the separate atoms? antibonding higher lower the same not enough information to decide σ Π х Garrow_forwardIn a diatomic molecule, a molecular orbital with electron density which is radially symmetrical about the bond axis, has e density in the bond axis, and pulls the molecule together is always shaped like a donut Which kind of MO always has a planar node? Ол On* O two of the abovearrow_forwardName the ten atomic orbitals that are used in the MO-theory to explain the overlap between S and F6.arrow_forward
- I have read that PH5 does not exist and was wondering why. Why can't the s orbitals from hydrogen form a bond with the five sp3d hybrid orbital from phosphorus? I've also read that PH4 does exist. Wouldn't the s orbitals on hydrogen form a bond with the sp3d hybrid orbitals there also with a free electron?arrow_forwardDraw an MO diagram to explain the existence of the trihydrogen cation (H3+). Hint: it forms a cyclic structure. Use individual 1s orbitals from H atoms to form your molecular orbitals (don’t worry about forming LGOs; determine the symmetries of the MOs using the projection operator method and sketch approximate wavefunctions for each). How many electrons are present in this molecule? What is the bond order? What type of bonding is this?arrow_forwardExplain why 1og is the ground state for Hi. By combining your answer with the answer to Problem 5, what conclu- sions can you draw about the molecular orbital descrip- tion of the bond in H¿?arrow_forward
- Consider a ring system made up of 6 atoms (each one contributing a p atomic orbital to make 6 pi molecular orbitals). If the system is filled with 8 electrons, there will ----orbitals containing pairs of electrons and------ orbitals containing single, unpaired electrons. Hint: Use a Frost Circle.arrow_forwardUse the template below to construct a MO diagram for the CN* molecule and use it to answer the following questions. In the completed diagram for CN*, the number of electrons in bonding orbitals is: in antibonding orbitals is: What is the bond order? If a fraction is needed, use a decimal number. CN* is Molecular orbitals Atomic orbitals (Atom A) Atomic orbitals (Atom B) 2p Encrgyarrow_forwardUsing the dropdown menu, answer the following questions based on the given pi molecular orbitals. Assume the compounds are simple hydrocarbons. 888 888888 1 2 3 888 8888 888 4 Is 1 an incorrect MO depiction? choose your answer... Are either 3 or 5 antibonding MOs? choose your answer... Are the energies of 3 and 5 equal? choose your answer... Which MO has the fewest nodes? choose your answer...arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY