
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
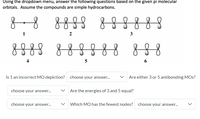
Transcribed Image Text:Using the dropdown menu, answer the following questions based on the given pi molecular
orbitals. Assume the compounds are simple hydrocarbons.
888
888888
1
2
3
888
8888 888
4
Is 1 an incorrect MO depiction?
choose your answer...
Are either 3 or 5 antibonding MOs?
choose your answer...
Are the energies of 3 and 5 equal?
choose your answer...
Which MO has the fewest nodes?
choose your answer...
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Construct the molecular orbital diagram for N,. Identify the bond order. 2p Answer Bank 1 11 2p 2p 0.5 1 1.5 2 2.5 * 3 2.s 2.sarrow_forward3. According to MO theory, which molecule or ion shown below has the highest bond order? Highest bond energy? Shortest bond strength? O2, C2-, O2^2- Draw MO energy diagrams for each of the species above and show your calculation or bond order. Also predict the magnetic properties in each case. Thank you very much.arrow_forwardht and are called the bonding and antibonding molecular orbitals. Which is higher in energy based on the nature of the electron density distribution? Which molecular orbital has a node and where?arrow_forward
- Y is a period two element with six valence electrons. i. Construct Molecular Orbital diagram for Y2*. ii. Identify the magnetism. i. Determine the bond order.arrow_forwardConstruct the molecular orbital diagram for H,. H H Answer Bank 11 1 ls 1s 1sarrow_forwarda. Construct the molecular orbital diagram for He2+2 and He2+ b. Find the bond order for both species. (numerical)arrow_forward
- Pls help ASAP, pls answer both questions I HUMBLY REQUEST.arrow_forwardDraw the orbital overlaps in the molecule C2H4S and label all orbitals in your sketch. For clarity you may choose to draw two diagrams to show the formation of sigma and pi bonds seperately.arrow_forward20. Determine the bond order in F2+ . Z of F = 9 a. 1 b. 3 c. 1.5 d. 3.5arrow_forward
- _ 2. In MO theory, combining two s atomic orbitals produces _____ and combining two p atomic orbitals produces ____. A. 2 sigma MOs; 6 sigma MOs B. 2 sigma MOs; 2 pi MOs C. 2 sigma MOs; 2 sigma MOs and 4 pi MOs D. 2 sigma MOs; 6 pi MOsarrow_forwardAA www-awu.aleks.com + Scoote G oxidation... my Tri-C... Content Learn finding di... n pe... O CHEMICAL BONDING Using the MO model to predict bond order and paramagnetism Heather v In particular: • Decide whether each molecule is stable or not. • Decide whether each molecule would be diamagnetic or paramagnetic. • Calculate each molecule's bond order. 圖 dlo diamagnetic or molecule stable? bond order paramagnetic? O yes O diamagnetic O no O paramagnetic O yes O diamagnetic Li, O no O paramagnetic O yes O diamagnetic N2 O no O paramagnetic Explanation Check O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use | Privacy | Accessibilityarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY